Abstract
The peripheral benzodiazepine receptor (PBR) is a mitochondrial protein involved in the formation of mitochondrial permeability transition (PT) pores which play a critical role during the early events of apoptosis. PBRs are located in many tissues and are strongly expressed in the superficial layers of human epidermis. PBRs play a protective role against free radical damage and PBR ligands modulate apoptosis. To investigate the role of PBR during the early events of ultraviolet (UV)-mediated apoptosis we compared the effects of UVB on PBR-transfected Jurkat cells and their wild type counterparts devoid of any PBR expression. Results indicate that early after UVB exposure (up to 4 h), PBR-transfected cells were more resistant to apoptosis and exhibited a delayed mitochondrial transmembrane potential drop, a diminished superoxide anions production, and a reduced caspase-3 activation. Taken together these findings suggest that PBR may regulate early death signals leading to UV induced apoptosis. Cell Death and Differentiation (2001) 8, 747–753
Similar content being viewed by others
Introduction
The peripheral benzodiazepine receptor (PBR) is a 18 kDa protein that is expressed on mitochondrial outer membranes, essentially distributed in peripheral tissues, and that is distinct from central benzodiazepine receptors.1,2 Endogenous ligands for PBRs include protoporphyrin IX and a newly described cytoplasmic protein, PRAX-1, that specifically interacts with these receptors.3,4 PBRs are involved in steroidogenesis, heme biosynthesis, immune and stress responses, cell growth, differentiation and mitochondrial respiratory control.5 Recent data also suggest that PBR is structurally and functionally related to the Rhodobacter sphaeroides tryptophan-rich sensory protein (TspO). Complementation experiments demonstrated that PBR was able to substitute for TspO taking part of a signal transduction pathway that regulates gene expression in response to oxygen.6,7 Within the mitochondrial membrane, PBR is closely associated with voltage-dependent anion channel (VDAC) and adenine nucleotide translocator (ANT) proteins which are involved in the formation of mitochondrial permeability transition (PT) pores.8 These multiprotein complexes play a critical role in apoptosis as opening PT pores and reduction of mitochondrial potential are the early irreversible steps of the programmed cell death (PCD) process.9,10,11,12,13 Interestingly, PBR has been shown to preserve the mitochondria of hematopoietic cell lines from damage induced by oxygen radicals14 and PBR ligands were found to modulate apoptosis.15,16 In human skin, as PBR expression is strongly upregulated in the superficial differentiated layers of the epidermis, those receptors are thought to play a protective role against free radical damage and apoptosis generated by ultraviolet (UV) light.17 In order to investigate the role of PBR during the early events of UV-mediated apoptosis, we analyzed the effects of UVB exposure on PBR-transfected Jurkat cells (PBR-J)14 compared to their wild-type counterparts (WT-J) devoid of any PBR expression.
Results
We choose the Jurkat cell lines because: (i) WT-J cells are naturally devoid of any PBR expression; (ii) and are non adherent cultured cells that do not require chemical or mechanical treatments which by themselves may modify the phosphatidylserine expression. Results of all the experiments were similar between clones 12-2-F8, 31-1-B10 and 12-2-D2 and there were no difference between Jurkat cell lines transformed with vector controls (pHb Neo APR1-J) and wild type Jurkat cells (WT-J). All the experiments were repeated thrice. χ2 and Wilcoxon tests were used for statistical analysis.
Analysis of early apoptosis induced by UVB treatment
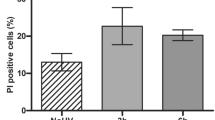
Cells were labeled with Annexin-FITC (A) and Propidium Iodide (PI). Apoptosis was first analyzed by flow cytometric analysis with increasing UVB doses (10–500 mj/cm2) at 1 h. A representative experiment is shown on Figure 1. Per cent of apoptotic but still viable cells (A+PI−) was reduced in PBR-J cells compared to WT-J cells with a dose-dependent effect. An optimal difference of apoptotic response between the two cell lines was observed with a 100 mJ/cm2 dose. Therefore we decided to study the kinetic of apoptosis at 1, 4, 12 and 24 h by using this irradiance. A representative experiment is shown on Figure 2a. Per cent of non viable PI+ (PI+ A+) cells increased after an acute UVB exposure in both cell lines. However, up to 4 h after UV treatment, the per cent of A+PI− cells was significantly reduced in PBR-J cells compared to WT-J cells (13% vs 44% at 1 h; 50% vs 75% at 4 h). At 12 h, most of cells have undergone apoptosis in both cell lines, the PI+ subset being still reduced in PBR-transfected cells (46% vs 60%). Such differences were confirmed from multiple experiments and there was no statistically significant difference between each tested PBR-transfected clones (Figure 2b,c). Furthermore, results were similar between wild type (WT-J) and cells transfected with an empty vector (pHb Neo APR1-J). At 1 h significant changes in early apoptosis were evidenced: WT-J cells exhibited a higher percentage of A+PI− cells than that of PBR cells (clone 12-2-F8: 34.1±6.17% versus 11±1.7%, P=0.001). At 4 h, such difference was also shown with a mean per cent of A+PI− cells of 51.9±2.66% in PBR-J cells (clone 12-2-F8) compared to that of 70.6±5.25% in WT-J cells (P=0.001) (Figure 2b). No significant loss of viability was observed after 1 and 4 h in wild type and PBR-transfected cell lines. Twelve hours after UVB exposure, the per cent of non viable A+PI+ cells in WT-J was higher (clone 12-2-F8: 63.3±3.5%) than that in PBR-J cells (47.0±2.65%) (P=0.001) while the per cent of A+PI− PBR-J cells (36.7±5.7%) was higher than that of WT-J cells (26±6%) (Figure 2c). At 24 h, most of A+ WT-J and PBR-J cells were PI+ (Figure 2a). These results indicate that early after UVB exposure (up to 4 h), PBR-transfected cells were more resistant to apoptosis than WT-J cells suggesting that PBRs may exert a transient protective effect during the initial events of programmed cell death.
UVB-induced apoptosis in WT-J and PBR-J cell lines. Flow cytometric analysis of WT-J and PBR-J cells (12-2-F8 clone) labeled with Annexin V (FITC) and propidium iodide (PI) were performed at 1 h after increasing UVB doses (10 to 500 mJ/cm2). In both cell lines per cent of A+PI− cells (apoptotic cells) increased in a dose-dependent manner. There were significantly less apoptotic cells (P=0.001) in PBR-J cells after 100 mJ/cm2 and 250 mJ/cm2 UVB exposures. The optimal difference between the apoptotic response of the two cell lines was obtained with a 100 mJ/cm2 dose. Results are representative of three different determinations
(a) UVB-induced apoptosis in WT-J and PBR-J cell lines. Flow cytometric analysis of WT-J and PBR-J cells (12-2-F8 clone) labeled with Annexin V (FITC) and propidium iodide (PI) were performed at 1, 4, 12 and 24 h after a UVB dose corresponding to 100 mJ/cm2. Up to 4 h, A+PI− cellular subsets (lower right quadrants) are reduced in PBR-J cells. After 12 h, per cent of A+PI− cells is greater in PBR-J cells (39% vs 31%) but PI+ subset is still reduced compared to WT-J cells (46% vs 60%). Results are representative of three independent experiments. Numbers refer to the percentage of cells contained in the indicated gate. (b) Kinetic study of apoptosis after UVB (100 mJ/cm2): at 1 and 4 h, apoptotic A+PI− cellular subsets are significantly reduced in PBR-J cells (P=0.001). Results are representative of three different determinations. No statistically significant difference was observed between 12-2-F8, 12-2-D2 and 31-1-B10 PBR transfected clones. The kinetic study was similar between Jurkat cells transfected with the corresponding empty vector (pHb Neo APR1-J) and the WT-J cells. (c) Kinetic study of apoptosis after UVB (100 mJ/cm2): at 12 h non viable A+PI+ cells are significantly reduced in PBR-J Jurkat cells (P=0.001). No statistically significant difference was observed between 12-2-F8, 12-2-D2 and 31-1-B10 PBR transfected clones. The kinetic study was similar between WT-J and pHb Neo APR1-J cells. Results are representative of three different determinations
Mitochondrial permeability transition after UVB exposure
In a number of experimental systems, the early stage of the apoptotic process is characterized by the breakdown of the inner mitochondrial transmembrane potential (ΔΨm).19 As PBR is part of the protein complex involved in the formation of mitochondrial permeability transition (PT) pores,8 we assessed the reduction of ΔΨm that occurs after UVB exposure (100 mJ/cm2) in WT and PBR-J cells. DiOC6 is a fluorochrome which incorporates into mitochondria in strict nonlinear dependence of ΔΨm and emits exclusively within the spectrum of green light. One representative experiment is shown in Figure 3a. One hour after UVB, 41% of WT-J cells became DiOC6low PI− whereas 54% remained PI− DiOC6high. By contrast the DiOC6low PI− cell subset was only 11% in PBR-J cells indicating that 1 h after UV, the release of DiOC6 was dramatically reduced in PBR-transfected cells. Until 4 h repeated experiments confirmed those results whereas at 12 and 24 h no statistically significant difference could be observed between the two cell types (Figure 3b). Taken together these results indicate that shortly following UV exposure, the ΔΨm drop is delayed in PBR-transfected cells compared to WT Jurkat cells.
(a) UVB induced ΔΨm drop in WT-J and PBR-J cell lines. Flow cytometric analysis of WT-J and PBR-J cells (12-2-F8 clone) labeled with DiOC6/PI 1 h after UVB exposure. Regarding PI expression, cells can be separated into PI−, PI+/− and PI+ populations. Gates were set on PI+ cells (upper left quadrants). After UVB, 41% of WT-J cells were DiOC6lowPI− whereas only 11% of PBR-J cells dropped their ΔΨm and became DiOC6low PI−. Results are representative of three independent experiments. (b) Kinetic study of ΔΨm drop in WT-J and PBR-J cells after UVB irradiation. DiOC6lowPI− cellular subsets represent cells that have released DiOC6 and dropped their ΔΨm. At 1 and 4 h after UVB exposure (100 mJ/cm2), there is a significant reduction of the DiOC6lowPI− subset in PBR-transfected Jurkat (PBR-J) cells (P=0.001). At 12 h, there is no statistically significant difference between the two types of cells. No statistically significant difference was observed between 12-2-F8, 12-2-D2 and 31-1-B10 PBR transfected clones. The kinetic study was similar between WT-J and pHb Neo APR1-J cells. Results are representative of three different experiments
Superoxide anions production after UV exposure
Reduction in ΔΨm and subsequent reactive oxygen species (ROS) production are observed in several in vitro models of programmed cell death. Early events of apoptosis that occurred before the nucleus or nuclear DNA are fragmented, included first a fall in ΔΨm followed by mitochondrial generation of ROS.20 To assess the production of superoxide anions, cells were labeled simultaneously with DiOC6 and oxidizing hydroethidine (HE), a substance that is oxidized by superoxide anions to produce ethidium and red fluorescence emission.21 A representative experiment is shown in Figure 4a. Flow cytometric analysis of WT and PBR-J cells (clone 12-2-F8) demonstrated that at each time point after UV exposure (1, 4 and 12 h), only DiOC6low cells produced superoxide anions. One hour after UV exposure, 44% of WT-J cells displayed a reduced ΔΨm (DiOC6low population in the two left quadrants) and almost 50% of them (21% of total number of cells) were Eth+. However, in PBR-J cells, only 8% of cells displayed a reduced ΔΨm and one third (2% of total cells) produced superoxide anions. Repeated experiments performed at 1, 4, 12 and 24 h (Figure 4b) indicate that until 4 h the superoxide anions production is significantly reduced in PBR-transfected cells (P=0.001).
(a) UVB induced ΔΨm drop and superoxide anions production in WT-J and PBR-J (12-2-F8 clone) cell lines. Flow cytometric analysis of WT-J and PBR-J cells were performed after UVB exposure. Cells were simultaneously stained with DiOC6 and HE (which allows for the determination of superoxide anion generation). Apoptotic DiOC6lowEth+ cellular subsets that produced ROS (upper left quadrants) are reduced in PBJ-J cells at 1 and 4 h after UV. Results are representative of three independent experiments. (b) Kinetic study of loss in ΔΨm and superoxide anions production in WT-J and PBR-J cells after UVB irradiation. At 1 and 4 h, superoxide anions production is significantly decreased in PBF-J cells (P=0.001). No statistically significant difference was observed between 12-2-F8, 12-2-D2 and 31-1-B10 PBR transfected clones. The kinetic study was similar between WT-J and pHb Neo APR1-J cells. Results are representative of three different determinations
Caspase-3 activation after UV exposure
UV irradiation induces activation of caspase 3 (CPP32-like protease) in the early stages of apoptosis.22 As mitochondria plays a critical role in the initiation of the caspase cascade,23 we measured the activity of caspase-3 in WT-J and PBR-J cells at 1, 2, 4, 12 and 24 h after UVB exposure (100 mJ/cm2). Shortly after irradiation, the activity of caspase-3 was increased in both WT and PBR-J cells up to 4 h, and returned to its baseline level at 24 h (Figure 5). At 2 and 4 h, caspase 3 activity was 20–50% higher in WT-J cells than that in PBR-J cells (P=0.01, Wilcoxon test). These results indicate that PBR expression is linked to a downregulation of caspase-3 activation that occurs in PBR-J cells shortly after UVB irradiation.
Kinetic study of caspase-3 activation after UVB exposure on PBR-J and WT-J cells. Jurkat cells were exposed to 100 mJ/cm2 UVB and incubated for 24 h at 37°C. Lysates were analyzed for caspase-3 activity and fluorescence (minus fluorescence of background) was plotted versus time. Caspase-3 activity is significantly decreased in PBR-J cells at 2 and 4 h (P=0.01). No statistically significant difference was observed between 12-2-F8, 12-2-D2 and 31-1-B10 PBR transfected clones. The kinetic study was similar between WT-J and pHb Neo APR1-J cells. Results are representative of three independent experiments
Discussion
Ultraviolet light (UV) generates singlet-oxygen damage and may trigger a PCD that does not require post-insult protein synthesis.24 This process may involve the PT pore which is a multiprotein complex formed at the contact site between the mitochondrial inner and outer membranes. The exact molecular composition of the pore is still unknown although several proteins have been described involved in mitochondrial PT pore formation such as VDAC (mitochondrial porine), ANT and PBR.8 Opening the PT pore can cause the dissipation of the inner mitochondrial transmembrane potential (ΔΨm) that is a critical event in the process leading to apoptosis.12,25,26 Moreover, recent evidence suggests that PT pore complex (PTPC) may constitute a crossroad of apoptosis regulation by caspases and members of the Bcl-2 family.19,27 Indeed, Bcl-2 proteins are particularly abundant in mitochondrial membrane where they are closely associated to PBRs and other proteins of the PTPC.10 Recent data demonstrate that proteins of the Bcl2 family bind to VDAC and regulate the mitochondrial membrane potential and the release of cytochrome c during apoptosis.11 Thus, a functional interaction between Bcl-2 and other components of PTPC such as PBR cannot be excluded. Interestingly, PK11195, a specific antagonistic ligand of PBR, facilitates the induction of apoptosis by triggering ΔΨm disruption and reverses Bcl-2 mediated cytoprotection.15,28 Moreover, Bcl-2 protects isolated mitochondria against the opening of the PT pore induced by low doses of protoporphyrin IX, a ligand of PBR.29 Mechanisms by which PBR and Bcl-2 exert anti-apoptotic effects are still not known and require further studies. Recent data demonstrate that a PBR agonistic ligand (Ro5-4864) is a potent anti-apoptotic compound.16 Finally the results reported here indicate that transfection-induced hyperexpression of the PBR gene protects against early UV-induced apoptosis as evidenced by a delay in mitochondrial changes and suggest that ΔΨm is stabilized by PBR. Altogether these data suggest a critical role for PBR in the apoptotic process.
Reactive oxygen species (ROS) are involved in many forms of PCD and in several in vitro models reduction of ΔΨm is followed by enhanced production of ROS and subsequent DNA fragmentation and nuclear DNA loss.20,30 UV-induced oxidative DNA damage is rapidly followed by the loss of ΔΨm and the activation of caspase-3, resulting in apoptosis.31 The demonstration of the role of PBR in the protection of hematopoietic cells against H2O2 suggests that PBR may prevent mitochondria from radical damage.14 Our findings are in agreement with these results and argue that PBR may be involved in ROS production induced by UV light. Mechanisms underlying these effects are still unclear but one may hypothesize that the reduced fall in ΔΨm due to PBR transfection results in a decreased generation of ROS that occurs shortly after UVB exposure. Indeed, PT pores participate in the generation of matrix Ca2+, pH, ΔΨm and redox-gated channel, and is implicated in cell death induced by anoxia, ROS, and calcium overload.27 Furthermore, opening the PT pores leads to the release of cytochrome c from mitochondria to the cytosol, thereby triggering caspases activation and cell death by apoptosis.32,33,34,35 Indeed, mitochondria are involved in the initiation of the caspase activation by releasing cytochrome c into the cytosol where it binds to the adaptator molecule Apaf-1 (apoptotic protease activating factor 1).35 This complex activates caspase-9 which then cleaves and activates downstream caspases such as caspase-3, -6, and -7. This pathway is regulated at several steps and caspase substrates such as Bcl-2 proteins appear to promote further caspase activation as part of a positive feedback loop.36 Caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins37 and initiating apoptotic DNA fragmentation.38 Recent data demonstrate that activation of caspase-3 is increased in the early stages of UVB-induced apoptosis.22,39 Our results indicate that caspase-3 activation after UVB is delayed in PBR-transfected cells. Whether this effect is related to protein-protein interactions within the mitochondrial PT pore or results from a disruption of the feedback loop control of caspase activation remains to be elucidated.33,40 Nevertheless, our results indicate that PBR, an important component of mitochondrial PT pore, may exert transient anti-apoptotic effects during the early events of UV-induced PCD. These findings are in agreement with pharmacological studies showing that PBR ligands may modulate apoptosis15,16,28 of hematopoietic cells as well as epithelial or hematological malignancies.41 Taken together, these observations provide compelling evidence indicating that PBR belongs to the protein family which may interact within the mitochondrial membrane to regulate death signals leading to apoptosis.
Materials and Methods
Materials
Stable transfectants PBJ-J (12-2-F8, 31-1-B10 and 12-2-D2 clones) and the corresponding WT-J cell lines and empty-vector transfected Jurkat cell lines (pHb Neo APR1-J) were kindly provided by Sanofi-Synthelabo (Montpellier, France).14 Quantitation of the number of PBR sites per cell was performed by flow cytometry using no conjugated anti-PBR MoAb and the QIFI assay (QIFIKIT; Dako).18 Clones 12-2-F8, 31-B-10 and 12-2-D2 expressed 90 000±10 000, 50 000±10 000 and 45 000±5000 PBR sites per cell respectively. WT-J and pHb Neo APR1-J cells were devoid of any PBR expression.14 RPMI 1640 medium, phosphate-buffered saline (PBS), pyruvic acid, and essential and nonessential amino acids solutions were obtained from Gibco BRL (Cergy-pontoise, France). Fetal calf serum (FCS), penicillin, streptomycin sulfate and glutamine were purchased at Boehringer Mannheim (Meylan, Claix, France).
Cell cultures and UVB irradiation
WT-J and PBJ-J cell lines were routinely cultured in RPMI 1640 culture medium supplemented with 10% heat-inactivated FCS, 4 mM glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin sulfate at 37°C under 5% CO2 in a humidified atmosphere. WT-J and PBJ-J cells were seeded in 35 mm Petri dishes (106 cells/well) in phenol red-free RPMI 1640 containing 10% FCS. Cells were exposed to UVB radiation (290–320 nm, doses ranged from 0 to 500 mJ/cm2) by using a UV 800 Waldman device (Reichstett, France). Power density was monitored before each experiment by using a Waldmann UV-Meter (Waldmann, Schwenningen, Germany). The source to target distance was 30 cm and the cell culture temperature was kept constant at 25°C during irradiation. Sham-irradiated samples were used as controls. After irradiation, the cells were incubated at standard culture conditions for the given time periods.
Flow cytometric detection of apoptosis
The UVB induced cell death was analyzed at 1, 4, 12, 24 h after irradiation using flow cytometric detection of phosphatidylserine expression with the Annexin V-FITC kit (Bender MedSystems, Vienna, Austria). Briefly, cells (5×105 cells) were washed twice in PBS and resuspended in 195 μl binding buffer (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) and then incubated for 10 min at room temperature with 5 μl annexinV-FITC. After wash, cells were resuspended in 190 μl binding buffer and Propidium Iodide were added to a final concentration of 1 μg/ml for flow cytometric analysis. Bivariate analysis was performed on a FACSCan (Becton Dickinson, Mountain View, USA) equipped with a 488 nm argon laser. A minimum of 10 000 cells per sample was recorded and stored in list mode files. Data analysis was performed using the CELLQuest software (Becton Dickinson, St. Louis, USA).
Cytofluorometric analysis of mitochondrial transmembrane potential (ΔΨm), and superoxide anions generation
After UVB irradiation, cells were incubated from 0 to 24 h at 37°C in the standard medium. Following published protocols19,20 the following fluorochromes were employed to determine different apoptosis-associated change: to assess ΔΨm and cell mortality, cells (5×105 cells/ml) were washed twice and incubated with 3,3′-dihexyloxacarbocyanine iodide (DiOC6), 40 nM in PBS; Molecular Probes Inc., Eugene, OR, USA) and propidium iodide (PI, 5 μg/ml). To measure ΔΨm and superoxide anions generation, cells (5×105 cells/ml) were washed twice and incubated with DiOC6 and dihydroethidine (HE; 2 μM, Molecular Probes Inc., Eugene, OR, USA) for 15 min at room temperature. After incubation, cells were washed and suspended in PBS, and then analyzed on a FACScan.
Measurement of caspase-3 activity
After a 100 mJ/cm2 UVB irradiation, cells were continuously incubated at 37°C for 1, 2, 4, 12 and 24 h in the standard medium. Caspase-3 activity was determined using a Fluorometric Immunosorbent Enzyme Assay kit (Boehringer Mannheim, Germany) according to the manufacturer's instructions. Briefly, cells (2×106 cells) were washed with ice cold PBS and suspended in 200 μl of lysis buffer for 1 min on ice. Cell lysates were centrifugated at 1800×g for 15 mn and supernatants were collected. The reaction was initiated by addition of Ac-DEVD-AFC (Z-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin) to anti-caspase 3-coated microtiter containing apoptotic extract. Product formation was measured by using a Hitachi F-2000 spectrofluorometer with excitation at 400 nm and emission at 505 nm.
Abbreviations
- A:
-
annexin V
- ANT:
-
adenine nucleotide translocator
- DiOC6:
-
3,3′-dihexyloxacarbocyanine iodide
- ΔΨm:
-
mitochondrial transmembrane potential
- HE:
-
dihydroethidine
- MPT:
-
mitochondrial permeability transition
- PBR:
-
peripheral benzodiazepine receptor
- PBR-J:
-
peripheral benzodiazepine receptor-transfected Jurkat cells
- PT:
-
permeability transition
- PTPC:
-
PT pore complex
- PCD:
-
programmed cell death
- PI:
-
Propidium Iodide
- ROS:
-
reactive oxygen species
- UVB:
-
ultraviolet B
- VDAC:
-
voltage-dependent anion channel
- WT-J:
-
wild type Jurkat cells
References
Braestrup C, Squires RF . 1977 Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding Proc. Natl. Acad. Sci. USA 74: 3805–3809
Anholt RRH, Pedersen PL, De Souza EB, Snyder SH . 1986 The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane J. Biol. Chem. 261: 576–583
Verna A, Nye J, Snyder SH . 1987 Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor Proc. Natl. Acad. Sci. USA 84: 2256–2260
Galiegue S, Jbilo O, Combes T, Bribes E, Carayon P, Le Fur G, Casellas P . 1999 Cloning and characterization of PRAX-1. A new protein that specifically interacts with the peripheral benzodiazepine receptor J. Biol. Chem. 274: 2938–2952
Krueger KE . 1995 Molecular and functional properties of mitochondrial benzodiazepine receptors Biochim. Biophys. Acta 1241: 453–470
Yeliseev AA, Krueger KE, Kaplan S . 1997 A mammalian mitochondrial drug receptor functions as a bacterial ‘oxygen’ sensor Proc. Natl. Acad. Sci. USA 94: 5101–5106
Yeliseev AA, Kaplan S . 2000 TspO of rhodobacter sphaeroides. A structural and functional model for the mammalian peripheral benzodiazepine receptor J. Biol. Chem. 275: 5657–5667
McEnery MW, Snowman AM, Trifiletti RR, Snyder SH . 1992 Isolation of the mitochondrial benzodiazepine receptor: association with voltage-dependent anion channel and the adenine nucleotide carrier Proc. Natl. Acad. Sci. USA 89: 3170–3174
Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J, Petit PX, Kroemer G . 1995 Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo J. Exp. Med. 181: 1661–1672
Kroemer G . 1997 The proto-oncogene Bcl-2 and its role in regulating apoptosis Nat. Med. 3: 614–620
Shimizu S, Narita M, Tsujimoto Y . 1999 Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC Nature 399: 483–487
Kroemer G, Zamzami N, Susin SA . 1997 Mitochondrial control of apoptosis Immunol. Today 18: 44–51
Bauer MK, Schubert A, Rocks O, Grimm S . 1999 Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis J. Cell. Biol. 147: 1493–1502
Carayon P, Portier M, Dussossoy D, Bord A, Petitpretre G, Canat X, Le Fur G, Casellas P . 1996 Involvement of peripheral bnezodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage Blood 87: 3170–3178
Hirsch T, Decaudin D, Susin SA, Marchetti P, Larochette N, Resche-Rigon M, Kroemer G . 1998 PK11195, a ligand of the mitochondrial benzodiazepine receptor, facilitates the induction of apoptosis and reverses Bcl-2-mediated cytoprotection Exp. Cell Res. 241: 426–434
Bono F, Lamarche I, Prabonnaud V, Le Fur G, Herbert JM . 1999 Peripheral benzodiazepine receptor agonists exhibit potent antiapoptotic activities Biochem. Biophys. Res. Commun. 265: 457–461
Stoebner PE, Carayon P, Penarier G, Frechin N, Barneon G, Casellas P, Cano JP, Meynadier J, Meunier L . 1999 The expression of peripheral benzodiazepine receptors in human skin: the relationship with epidermal cell differentiation Br. J. Dermatol. 140: 1010–1016
Dussossoy D, Carayon P, Feraut D, Belugou S, Combes T, Canat X, Vidal H, Casellas P . 1996 Development of a monoclonal antibody to immuno-cytochemical analysis of the cellular localization of the peripheral benzodiazepine receptor Cytometry 24: 39–48
Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G . 1996 Mitochondrial permeability transition is a central coordinating event of apoptosis J. Exp. Med. 184: 1155–1160
Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G . 1995 Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death J. Exp. Med. 182: 367–377
Rothe G, Valet G . 1990 Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin J. Leukoc. Biol. 47: 440–448
Shimizu H, Banno Y, Sumi N, Naganawa T, Kitajima Y, Nozawa Y . 1999 Activation of p38 mitogen-activated protein kinase and caspases in UVB-induced apoptosis of human keratinocyte HaCat cells J. Invest. Dermatol. 112: 769–774
Wolf BB, Green DR . 1999 Suicidal tendencies: apoptotic cell death by caspase family proteinases J. Biol. Chem. 274: 20049–20052
Godar DE . 1999 Light and death: photons and apoptosis J. Investig. Dermatol. Symp. Proc. 4: 17–23
Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G . 1996 Mitochondrial control of nuclear apoptosis J. Exp. Med. 183: 1533–1544
Green DR, Reed JC . 1998 Mitochondria and apoptosis Science 281: 1309–1312
Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC, Kroemer G . 1998 The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins J. Exp. Med. 187: 1261–1271
Tanimoto Y, Onishi Y, Sato Y, Kizaki H . 1999 Benzodiazepine receptor agonists modulate thymocyte apoptosis through reduction of the mitochondrial transmembrane potential Jpn. J. Pharmacol. 79: 177–183
Marchetti P, Hirsch T, Zamzami N, Castedo M, Decaudin D, Susin SA, Massa B, Kroemer G . 1996 Mitochondrial permeability transition triggers lymphocyte apoptosis J. Immunol. 157: 4830–4836
Tan S, Sagara Y, Liu Y, Maher P, Schubert D . 1998 The regulation of reactive oxygen species production during programmed cell death J. Cell. Biol. 141: 1423–1432
Tada-Oikawa S, Oikawa S, Kawanishi S . 1998 Role of ultraviolet A-induced oxidative DNA damage in apoptosis via loss of mitochondrial membrane potential and caspase-3 activation Biochem. Biophys. Res. Commun. 247: 693–696
Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR . 2000 The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant Nat. Cell Biol. 2: 156–162
Chen Q, Gong B, Almasan A . 2000 Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis Cell Death Differ. 7: 227–233
Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prevost MC, Leber B, Andrews D, Penninger J, Kroemer G . 2000 Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis FASEB J. 14: 729–739
Bossy-Wetzel E, Green DR . 1999 Caspases induce cytochrome c release from mitochondria by activating cytosolic factors J. Biol. Chem. 274: 17484–17490
Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM . 1999 Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c J. Biol. Chem. 274: 21155–21161
Porter AG, Janicke RU . 1999 Emerging roles of caspase-3 in apoptosis Cell Death Differ. 6: 99–104
Wolf BB, Schuler M, Echeverri F, Green DR . 1999 Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation J. Biol. Chem. 274: 30651–30656
Aragane Y, Kulms D, Metze D, Wilkes G, Poppelmann B. Luger TA, Schwarz T . 1998 Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L J. Cell. Biol. 140: 171–182
Slee EA, Keogh SA, Martin SJ . 2000 Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release Cell Death Differ. 7: 556–565
Xia W, Spector S, Hardy L, Zhao S, Saluk A, Alemane L, Spector NL . 2000 Tumor selective G2/M cell cycle arrest and apoptosis of epithelial and hematological malignancies by BBL22, a benzazepine Proc. Natl. Acad. Sci. USA 97: 7494–7499
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Salvesen
Rights and permissions
About this article
Cite this article
Stoebner, PE., Carayon, P., Casellas, P. et al. Transient protection by peripheral benzodiazepine receptors during the early events of ultraviolet light-induced apoptosis. Cell Death Differ 8, 747–753 (2001). https://doi.org/10.1038/sj.cdd.4400861
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400861
Keywords
This article is cited by
-
The nitric oxide donor sodium nitroprusside requires the 18 kDa Translocator Protein to induce cell death
Apoptosis (2012)
-
Role of translocator protein in melanoma growth and progression
Archives of Dermatological Research (2012)
-
Mitochondria as therapeutic targets for cancer chemotherapy
Oncogene (2006)
-
Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario
Cell Death & Differentiation (2006)
-
Chemotherapy: targeting the mitochondrial cell death pathway
Oncogene (2002)