Abstract
Owing to its lineage and differentiation stage-restricted expression, CD77 has been mooted as a therapeutic target in Burkitt lymphoma (BL). The recognition that the globotriaosyl moiety of this neutral glycosphingolipid is a receptor for Escherichia coli-derived Verotoxin-1 (Shiga-Like Toxin-1) offers a potential delivery system for the attack. Here we show that CD77-expressing Group I BL cells which are normally susceptible to activation-induced death on binding Verotoxin-1 B chain are protected in the presence of CD40 ligand. Ectopic expression of either bcl-2 or bcl-xL also afforded resistance to the actions of the B chain. In total contrast, neither of the survival genes nor a CD40 signal – even when acting in concert – protected against killing mediated by the holotoxin. These findings indicate that while therapeutic modalities for CD77-expressing B cell tumors (which include follicular lymphoma) based on the use of Verotoxin-1 B chain might be compromised by the activation of endogenous or exogenous survival pathways, those exploiting the holotoxin should be left unscathed. Cell Death and Differentiation (2000) 7, 785–794
Similar content being viewed by others
Introduction
Verotoxin-1 (VT-1) produced by the enteropathogenic O157 strain of Escherichia coli binds selectively to the α-D-gal-(1→4)-β-D-gal-(1→4)-β-D-glucose-(1→) trisaccharide moiety of globotriaosylceramide, also known as CD77.1,2,3,4 Expressed on subsets of endothelial, mesangial, and epithelial cells, the surface representation of CD77 on hematopoietic cells – with the exception of subtypes of erythrocytes and some low level expression on monocytes – is restricted to centroblasts, cells that constitute the proliferating B cell compartment of germinal centers (GC) located within secondary lymphoid organs.5,6,7,8,9,10,11 From its cell line-restricted reactivity and an inability to bind recirculating normal B cells, the prototype CD77 mAb, 38.13, was initially designated as defining a moiety termed ‘BLA’, or Burkitt Lymphoma Antigen.12 Based partly on their mutual expression of CD77, BL cells are now deemed to represent phenotypic tumor equivalents of normal GC B cells.13,14 A large proportion of follicular lymphoma also appears to remain faithful to their normal counterparts by displaying CD77 on the tumor cell surface.15
VT-1 is composed of an A subunit non-covalently associated with a pentamer of B subunits. The A subunit itself is further divided into a catalytic A1 region and a B chain-binding A2 region by a furin cleavage site. Cleavage at this site is important for optimal activation of toxin during cellular uptake.16 CD77 permits the cellular entry of VT-1 through an initial binding of the pentameric B subunits of the holotoxin. Subsequent endocytosis of toxin to the endoplasmic reticulum, and membrane translocation to the cytosol, results in the inactivation of ribosomes by the toxin's catalytic A1 subunit. This inactivation involves a specific depurination of 28S ribosomal RNA which renders the ribosomes unable to function in protein synthesis: an event which ultimately leads to the death of the cell.16,17 VT-1 has thereby been mooted as a potential delivery system for toxin-based therapy of CD77-expressing lymphoma: indeed, a range of BL cell lines have been shown to die on exposure to the holotoxin in culture.9,18 In addition to the ribosome-inactivating properties of the A chain, it was found that B chain pentamer – when present at sufficiently high concentration – was, of itself, able to deliver a death signal to BL cells.18 Subsequent studies using the 38.13 antibody indicated that the cross-linking of CD77 initiated an intracellular signaling cascade (involving a rise in cytosolic Ca2+ and the generation of ceramide) which culminated in the induction of apoptosis.9,19 Thus it appeared that therapeutic attack on CD77-expressing lymphoma independent of VT-1 A chain might now be feasible.
Normal GC B cells display both in situ and in vitro a high propensity for spontaneous apoptosis, a process reflecting the need to select for high affinity mutations that target the V region genes in proliferating centroblasts.11 The constitutive B cells of GC fail to express the pro-survival protein Bcl-2, a feature retained by BL cells.11,20,21,22,23,24 Notably, BL is characterized by a classical ‘starry sky’ histology reflecting the presence and activity of tingible body macrophages that normally colonize GC in order to manage the high rate apoptosis occurring at these sites.11,25,26,27 Moreover, BL cell lines in early passage that remain faithful to the biopsy phenotype (termed ‘group I’) are both Bcl-2-negative and can be readily prompted into apoptosis by a variety of signals including those induced by antigen-receptor cross-linking.23,28,29,30,31
Although BL arising in endemic areas invariably harbor Epstein-Barr virus (EBV), freshly-isolated tumor cells and group I lines derived from them fail to express EBV genes involved in B cell immortalization.32,33,34 On long-term culture, however, such lines display ‘phenotypic drift’ and ultimately give rise to group III lines as a result of full expression of EBV transforming genes.33,34 This is accompanied by the appearance of Bcl-2 protein and a subsequent resistance to antigen-receptor-dependent apoptosis.23,29 Introduction of bcl-2 directly into group I cells by gene transfection has confirmed the central importance of Bcl-2 to the anti-apoptotic phenotype that emerges.22,23 Follicular lymphoma is quite different from BL being characterized by intrinsic high survival driven by the influence of deregulated bcl-2 that arises from the 14 : 18 chromosomal translocation that defines around 90% of cases.35,36 This wired-in survival pathway may underlie the observed difficulty in eradicating the tumor population in this disease.37
Other survival mechanisms for normal and lymphoma B cells exist. For example, the bcl-2 related gene bcl-xL can offer protection against apoptosis in some scenarios.38,39,40,41 Extracellular signals can also engender protection. For both normal GC B cells and BL cell lines, the most potent of these is provided by CD40 ligand (CD40L) activating its receptor, CD40, on the target cell.20,21,29,31 CD40L – a member of the TNF family – has been characterized as an inducible surface molecule of T-helper cells although a number of studies are now indicating its presence on at least subsets of normal and malignant B cells.42,43,44,45 Thus B lymphoma cells may receive survival prompts both from infiltrating T cells and, potentially, via autocrine/juxtacrine modes.
The design of therapeutic strategies should take account of both the intrinsic and extrinsic means by which a tumor could circumvent the desired result: namely, cell death. Using BL as the model for CD77-expressing lymphoma, we have explored the potential impact of the known survival pathways exploited by B cells on their response to VT-1. The findings demonstrate that these would succeed in protecting lymphoma cells from VT-1 B chain. They would, however, be thwarted when eliciting A chain-dependent killing with the holotoxin.
Results
CD77-dependent sensitivity to cell death is VT-specific
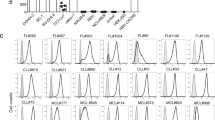
Physiological expression of CD77 on B lymphocytes is restricted to those with an apoptotic phenotype leading to the suggestion that its presence may be an intrinsic component of their capacity for programmed cell death (PCD).9,46 We assessed here whether the presence of CD77 on BL lines was requisite to their ability to undergo activation-induced PCD while attempting to confirm its necessity for VT-1-mediated killing. This was facilitated by the observation that the L3055 group I BL line maintained in early passage comprised a dual population of CD77pos and CD77neg cells at an approximate 2 : 1 ratio (Figure 1a). Exposure of this line to 100 ng/ml VT-1 for 48 h led to the disappearance of the CD77pos fraction leaving a viable population that was exclusively CD77neg (Figure 1b). It should be noted that with increasing passage, the percentage of CD77pos cells in untreated L3055 cultures fell steadily from around 70% in early passages (<30) to approximately 30% in later ones (>60). The growing proportion of cells that remained viable following exposure to VT-1 closely reflected the increasing number of CD77neg cells present as the cultures progressed (data not detailed). This not only highlights the specificity of VT-1 for CD77-expressing cells but also indicates that the CD77neg population emerging post-VT-1 exposure did not arise simply as a consequence of globotriaosylceramide down-regulation at the cell surface: this conclusion was supported by the finding that VT-1 treatment of a group I line where cells were uniformly CD77pos (Mutu I) failed to yield a viable CD77neg subpopulation (n=4).
Selection of VT-1-resistant L3055 cells. L3055 wild-type cells (a,c) were cultured for 48 h with 100 ng/ml VT-1 after which the viable cells were recovered (b,d). Cells were stained for CD77 expression (a,b) with directly-conjugated 38.13 mAb (open histograms) or rat IgM control (shaded histograms). The response of the cells to subsequent culture with VT-1 was monitored by DNA synthesis (c,d) as assessed by 3HTdr incorporation at 24 (♦) or 48 h (▪). Results expressed as means of quadruplicate determinations (S.E.M. always <10% of mean values) are representative of four similar experiments
When assessed for its ability to respond to VT-1 by the cessation of DNA synthesis (both an indicator of cell killing and a prelude to apoptosis for these cells24,31) the wild-type population displayed a dose-dependent inhibition that by 24 h reached a maximum of around 70% with VT-1 concentrations of 10 ng/ml and above (Figure 1c). Extending exposure to 48 h failed to increase significantly the extent of this inhibition. By contrast, the VT-1 selected CD77neg cells were resistant to VT-1-dependent inhibition whether measured at 24 or 48 h (Figure 1d). While the CD77neg cells were similarly refractory to cessation of DNA synthesis promoted by VT-1 B chain, they remained fully sensitive to the inhibitory effects of cross-linking antigen receptor, elicited here by antibody (BU1) to sIgM (data not detailed). The outcome of these treatments as revealed by the inhibition of DNA synthesis was mirrored in a loss of cell viability and/or the appearance of apoptotic nuclei as assessed by visualizing acridine orange-stained cells (Table 1). The latter measurement was significant only for cells exposed to anti-IgM or B chain: the overwhelming majority of cells treated with VT-1 holotoxin had the appearance of lysis or necrosis rather than apoptosis. These data reveal that resistance to killing of group I BL lines resulting from a loss of CD77 is exclusive to VT-mediated pathways.
Expression of bcl-2 and bcl-xL confer resistance to VT-1 B chain but fail to protect from holotoxin
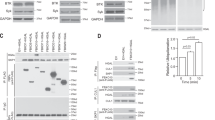
L3055 cells were forced to express the bcl-2 or bcl-xL genes by generating stable transfectants under the influence of the human EF-1α promoter. A clone transfected with empty vector was established as a control. Another group I BL line, Mutu I, was used to establish a similar set of transfectants. Unlike L3055 cells, Mutu is an EBV-harboring BL line that on long term culture progresses to a group III phenotype.29,34 The Mutu III line was also included for study. The status of Bcl-2 and Bcl-xL expression among the different lines was confirmed by Western blotting as illustrated in Figure 2. It should be noted that the Mutu III cells contained a significant level of constitutively expressed Bcl-2 protein albeit lower than that found in the bcl-2-transfected lines.
Expression of Bcl-2 and Bcl-xL in L3055 and Mutu lines. Cell lysates were prepared from pelleted cells and equal amounts of total cellular protein were resolved on 12.5% SDS–PAGE. Western blotting was performed to detect the presence of Bcl-2 (lower panel) and Bcl-xL (upper panel) with relative molecular weights by reference to standard markers indicated
Results presented in Figure 3 show that both Bcl-2 and, to a lesser but still significant extent, Bcl-xL protected L3055 cells from cessation of DNA synthesis induced by antibody to sIgM. They each similarly protected from the inhibitory actions of VT-1 B chain. When assessed for sensitivity to VT-1 holotoxin, neither Bcl-2 nor Bcl-xL was seen to afford protection to L3055 cells. The clone selected for the control vector showed a somewhat greater susceptibility than that of wild-type L3055 cells to both VT-1 B chain and holotoxin (Figure 3).
Inhibition of DNA synthesis in L3055 transfectants in response to treatments. Cells were plated with concentrations of: (a) BU1 (anti-IgM); (b) VT-1 B chain; or (c) VT-1 holotoxin as indicated and DNA synthesis assessed at 48 h as in Figure 1. Results are expressed as percentage of control 3HTdr incorporation relative to culture in control medium alone. In this experiment (which is representative of three) the absolute values (as c.p.m. with S.E.M. in parentheses) were: L3 wild-type (♦), 53 257 (3189); L3 vector control (▪), 66 209 (2855); L3/Bcl-2 (▴), 86 340 (4002); L3/Bcl-xL (•) 75 716 (4076). S.E.M. were always <10% of mean values
While Mutu I cells are relatively resistant to growth inhibition and programmed death mediated via soluble anti-IgM (unpublished observation) they can be encouraged into apoptosis by the calcium ionophore, ionomycin.29 It can be seen from Figure 4 that Bcl-2 and Bcl-xL each afforded protection to Mutu I cells from the effects not only of ionomycin but also of VT-1 B chain. Neither Bcl-2 nor Bcl-xL was able to offer resistance to Mutu I cells from VT-1 holotoxin (Figure 4). Mutu III cells displayed a modest cessation of DNA synthesis in response to holotoxin but were fully resistant to both VT-1 B chain and the calcium ionophore.
Inhibition of DNA synthesis in Mutu I transfectants and Mutu III cells in response to treatments. As for Figure 3 except cells were plated with concentrations of : (a) ionomycin; (b) VT-1 B chain; or (c) VT-1 holotoxin. In this experiment (representative of three) the absolute values (as c.p.m. with S.E.M. in parentheses) of 3HTdr incorporation in culture medium alone were: Mutu I wild-type (♦), 65 049 (2832); Mutu I vector control (▪), 62 477 (3509); Mutu I/Bcl-2 (▴), 99 404 (5878); Mutu I/Bcl-xL (•), 85 110 (1694); Mutu III (⋄), 72 565 (4902). S.E.M. were always <10% of mean values
Inhibition of DNA synthesis in Mutu I transfectants and Mutu III cells in response to treatments. As for Figure 3 except cells were plated with concentrations of : (a) ionomycin; (b) VT-1 B chain; or (c) VT-1 holotoxin. In this experiment (representative of three) the absolute values (as c.p.m. with S.E.M. in parentheses) of 3HTdr incorporation in culture medium alone were: Mutu I wild-type (♦), 65 049 (2832); Mutu I vector control (▪), 62 477 (3509); Mutu I/Bcl-2 (▴), 99 404 (5878); Mutu I/Bcl-xL (•), 85 110 (1694); Mutu III (⋄), 72 565 (4902). S.E.M. were always <10% of mean values
Effects monitored by cessation of DNA synthesis were again reflected in the appearance of death and/or apoptotic cells (Figure 5). These results confirmed the protection afforded by Bcl-2 and Bcl-xL to L3055 cells from apoptotic death induced by cross-linking sIgM and following exposure to VT-1 B chain. Death still ensued on exposure to VT-1 holotoxin however. Bcl-2 and Bcl-xL were again seen to protect Mutu I cells from calcium ionophore-induced death and that engendered by VT-1 B chain but failed to protect from killing mediated via holotoxin. Apart from a minor – and variable – population that was susceptible to the holotoxin, Mutu III cells were essentially resistant to all the routes of cell death (Figure 5 and see below). It should be noted that with concentrations of VT-1 holotoxin that were sub-optimal, in that they elicited only partial killing of the populations, the death that ensued – as exemplified here for the L3055 series – remained primarily non-apoptotic irrespective of survival gene status (Table 2).
Survival genes protect from B chain- but not VT-1 holotoxin-induced death. Cells were cultured for 48 h in the presence of: control medium (CM); BU1 (anti-IgM, 500 ng/ml); calcium ionophore, ionomycin (CaI, 1 μg/ml); VT-1 B chain (B chain, 100 μg/ml); or VT-1 holotoxin (VT-1, 30 ng/ml) as indicated. (a) L3055 series; (b) Mutu series. The percentage of dead or apoptotic cells arising following treatments was enumerated as for Table 1. Results given are the means of three separate experiments: S.D. were never >17% and error bars are omitted for clarity
When assessed for CD77 expression, all lines were clearly positive with the exception of Mutu III cells where a minority of cells stained weakly only: the disappearance of CD77 is a hallmark characteristic of a group I to group III transition among BL lines.13,34 Interestingly, the bimodal distribution of CD77 expression seen with the L3055 wild-type cells was retained in both the bcl-2 and bcl-xL transfectants while the vector controls displayed a uniformly CD77high population (data not detailed). All Mutu I transfectants revealed a unimodal high level staining for CD77 (see, for example, Figure 7).
Influence of sCD40L on CD77 expression. Mutu I vector control cells were cultured for 1 or 2 days with sCD40L (50 ng/ml) as indicated. CD77 expression was then assessed by indirect staining with 38.13 mAb (open histograms) or a rat IgM control (shaded histograms). Data are representative of three similar experiments
Thus, among the different lines studied, there was a close correlation between the level of CD77 positivity and susceptibility to VT-1 holotoxin. This association was highlighted by the Mutu III line where the number of dead cells arising in response to VT-1 exposure mirrored closely the proportion of CD77pos cells detected at the time of analysis – thus, over four experiments, the following percentages for ‘death’ (as measured by trypan blue dye uptake following 24 h treatment with VT-1) versus ‘CD77-positivity’ (determined by FACS analysis at t=0) were observed: (i) 13% vs 10%; (ii) 26% vs 25%; (iii) 27% vs 24%; (iv) 43% vs 39%. The reason for the variability in CD77 expression seen with Mutu III cells is presently unclear but may relate to the growth phase of the cultures (our own unpublished observations).
CD40 signals discriminate against B chain- and holotoxin-mediated death pathways
The presence of soluble CD40L at 50 ng/ml protected both L3055 and Mutu I wild-type cells from cessation of DNA synthesis mediated by soluble anti-IgM and ionomycin respectively, confirming previous observations.21,24,29 CD40L offered similar protection from VT-1 B-chain but left the actions of VT-1 holotoxin unchecked. CD40 signaling failed to protect against the holotoxin even for lines expressing bcl-2 or bcl-xL (data not detailed). Assessments of cell viability once more confirmed these observations (Figure 6). On studying SAV and BL2 – two other CD77pos group I BL lines sensitive to sIgM-dependent apoptosis – there was an identical outcome in the ability of CD40L to protect from death mediated by soluble anti-IgM or VT-1 B-chain while failing to spare them from killing elicited by the holotoxin (Table 3).
CD40L protects from B chain- but not VT-1-holotoxin-induced death. As for Figure 6 but results presented as percentage viable cells remaining after 48 h. Treatments are indicated without (−) or with (+) sCD40L present at 50 ng/ml. (a) L3055 series; (b) Mutu series. Results given are the means of three separate experiments: S.D. were never >17% and error bars are omitted for clarity
Finally, we asked whether CD40L might be offering protection to the cells from B chain killing by down-regulating CD77 expression, a phenomenon previously reported for group I cells exposed to high concentrations (1 μg/ml) of soluble CD40L for 3 days or more.24 While cells cultured with CD40L remained sensitive to death promoted by holotoxin, the need for extensive cross-linking of CD77 to elicit A chain-independent killing via CD77 might necessitate that high level receptor expression is maintained for B chain-mediated apoptosis to proceed.9,19 Mutu I cells were used for these studies as they displayed a uniformly high CD77 starting population. It is clear from the results presented in Figure 7 that culture of Mutu I cells (here shown for vector controls) with 50 ng/ml of soluble CD40L for either 24 or 48 h did not lead to a disappearance of CD77. Although CD77 levels did start to fall by day 2, expression remained substantive and was unlikely to be limiting: indeed, even after incubation with soluble CD40L, Mutu I vector controls carried higher levels of CD77 than wild-type cells. A similar outcome was obtained for Mutu I cells carrying bcl-2 or bcl-xL (data not detailed).
Discussion
In certain respects, group I BL forced to express bcl-2 ectopically models follicular lymphoma. Both B cell tumors display a GC phenotype with the exception of follicular lymphoma being constitutively Bcl-2 positive due to the deregulation of the bcl-2 gene arising from the 14 : 18 translocation that characterizes this disease.14,15,35,36 For any therapeutic modality to succeed against this most common of the lymphoma, it needs to overcome the powerful survival influence afforded to B cells by Bcl-2. Moreover, normally Bcl-2 negative BL cells can be prompted by extracellular signals to express the survival protein.24 To confound matters further, CD40L can stimulate survival pathways in normal and malignant B cells independently of bcl-2.21 For both human and murine B cells, CD40 engagement can lead to an upregulation of the anti-apoptotic bcl-2 family member, bcl-xL38,39,40,41 Though yet to be proven, the potential for CD40 signaling in B cell lymphoma exists by way of infiltrating T cells and via autocrine/juxtracrine CD40L-CD40 interactions.43,44,45
Here we have shown that while activation of either endogenous or exogenous survival pathways spares BL cells from death mediated by the B chain of VT-1, they remain fully susceptible to A chain-dependent killing elicited by VT-1 holotoxin. This extended to cells both ectopically expressing high levels of survival genes and receiving CD40L signals, an important outcome given the finding of Ghia et al that CD40-stimulated up-regulation of bcl-xL in follicular lymphoma may contribute to enhanced survival.47 Moreover, we have recently reported that normal, Bcl-2 positive, non-germinal center B cells induced to express CD77 by high level engagement of CD40L gain full susceptibility to killing by the holotoxin.48 The complete resistance to B chain killing exhibited by the Mutu III cell line, while remaining fully susceptible to holotoxin, may have been due to more than the relatively low level of Bcl-2 that was found to be expressed: the EBV-encoded Latent Membrane Protein-1 (LMP-1) protein that is turned on as group I cells progress towards a group III phenotype additionally activates the expression of a zinc finger protein (A20) that can provide independent signaling for cell survival:49,50 moreover, LMP-1 has been shown to behave – in many respects – as a constitutively active CD40 molecule providing further bcl-2-independent, anti-apoptotic potential when expressed.51
In toto, the above indicate that although therapeutic exploitation of VT-1 B chain for CD77-expressing lymphoma would seemingly have only limited application, that of VT-1 holotoxin appears to hold more universal appeal.
The capacity of the survival pathways to discriminate against B chain- and A chain-dependent killing is likely to reflect the different modes of cell death elicited by the two. High concentrations of pentameric B chain are required to achieve killing of group I BL cells [Mangeney et al18 and data reported herein]. The ensuing cell death has been described previously as arising from an apoptotic pathway.18 This we confirmed by demonstrating the generation of apoptotic nuclei as revealed by visualization of acridine orange-stained cells. Moreover, the ability to protect against this route of killing by anti-apoptotic signals in itself reinforces the notion that B chain triggers programmed death in these cells. It was recently reported that on cross-linking 38.13 antibody bound to CD77, an intracellular signaling cascade is initiated involving a rise in cytosolic Ca2+ and the generation of ceramide.9,19 Boht have been implicated as components of apoptotic cell death in B lymphoma cells.28,52
The A chain of VT-1 promotes cell death by inhibiting protein synthesis as a consequence of modifying ribosomal RNA.16,17 While still requiring the B chain to gain entry into a target cell, it is questionable whether CD77-dependent pathways additional to those of simple binding and endocytosis are operative in killing elicited by the holotoxin. This statement is based on the observation that VT-1 holotoxin kills cells at concentrations considerably less (<10 ng/ml) than those required for B chain to be active (>10 μg/ml). The involvement of an apoptotic component to holotoxin-mediated death is not ruled out however. It was recently shown that Pseudomonas endotoxin (PE) – which similar to VT-1 can kill target cells via A chain-dependent inhibition of protein synthesis – also increases caspase activity and induces PARP cleavage in a neoplastic epithelial cell line.53 Thus, like PE, VT-1 may be exploiting two routes to cell death. Indeed, although for the lines used in the present study we were unable to detect significant induction of fragmented nuclei in response to the holotoxin, apoptotic morphology has been reported for other BL cells exposed to low doses of VT-1.18 Despite this, as evidenced from the inability of either CD40 signals, Bcl-2, or Bcl-xL to protect, it seems that even though VT-1 A chain might be capable of stimulating components of apoptosis, this pathway is redundant to the ultimate outcome: i.e., the killing of the cell. A similar conclusion was reached from a study on the VT-1 killing of endothelial cells where caspase inhibitors were able to reverse the measures of apoptosis made while death proceeded as normal.54
For group I BL cells, CD77 was found not to be requisite for the expression of an apoptotic phenotype per se. This possibility had been raised previously in regard to both BL and their normal GC B cell equivalents:9,46 indeed, a mechanistic basis for this could be envisaged through a potential pro-apoptotic activity being generated from the intracellular ceramide moiety of CD77. Nevertheless, while the CD77neg fraction of the L3055 line was clearly refractory to killing via both VT-1 B chain and holotoxin they remained fully susceptible to apoptosis induced by cross-linking their sIgM. The retention of an apoptotic capacity by VT-1-selected CD77neg cells is encouraging for the outcome of any VT-based therapy where similar escape variants could potentially arise on treating patients with the toxin in vivo.
Having established a preference for exploiting the holotoxin in any CD77-targeted attack on B cell lymphoma, consideration must be given to the danger of damaging other cells, especially CD77pos endothelial cells of the kidney. Verotoxin-producing Escherichia coli strains have been strongly implicated in the etiology of hemolytic uremic syndrome, the leading cause of pediatric acute renal failure.55,56 However, reflecting the age distribution of this problem, some comfort is given by the finding that VT-binding, while it can be observed in the infant glomerulus, is not detectable in the adult where, instead, it is restricted to tubules.5 We are currently addressing whether even lower concentrations of VT-1 than those used in the present study might act in synergy with other B cell-directed therapeutics and thereby minimize any deleterious effects on non-B cells. CD20 antibodies, already being used with promise in follicular lymphoma, are a consideration here.57 Finally, we could consider mutating the VT-1 A chain to eliminate the furin-cleavage site which is important for A chain activation during cell cycle entry16 and to substitute alternative residues that would be susceptible to B cell-specific enzymes. Our finding that VT-1 holotoxin kills lymphoma cells even when confronted with powerful survival factors indicates that these possibilities warrant serious attention.
Materials and Methods
Reagents
The BU1 mouse IgG2a monoclonal antibody to human IgM was produced from the hybridoma in the Department of Immunology, University of Birmingham, and purified by ion-exchange chromatography on DE52 (Whatman Ltd, Maidstone, UK). Asitic fluid containing rat IgM anti-CD77 mAb (38.13) was a kind gift from Dr J Wiels (Institut Gustav-Roussy, Villejuif, France) and control ascitic fluid containing rat IgM anti-DNP was obtained from Serotec (Oxford, UK). 38.13 ascitic fluid and control rat IgM ascitic fluid were conjugated to FITC by standard techniques. In some experiments, unconjugated 38.13 mAb purchased from Serotec was used. Ionomycin was obtained from Calbiochem-Novabiochem (Nottingham, UK). Soluble trimeric CD40L was generated as an isoleucine zipper construct as described by Morris et al.58
Purification of Verotoxin-1 and VT-1 B chain
Verotoxin-1 was prepared from periplasmic extracts of E. coli JM105 transformed with the expression plasmid pSLT (a plasmid encoding the Verotoxin-1 operon under the control of the lac promoter). Cells were grown in Luria broth supplemented with 100 μg/ml ampicillin to an OD600 of 0.6 (measured with a Shimadzu UV-160 spectrophotometer). Expression was induced by the addition of isopropyl β-D-thiogalactopyranoside (1 mM final concentration) and allowed to proceed for 3 h. Cells were harvested by centrifugation (10 min at 7500×g), washed in 600 mM sucrose, 300 mM Tris/HCl (pH 8.0), 1 mM EDTA and 0.5 mM MgCl2, and resuspended in 1 mM Tris/HCl, pH 7.5. After incubation on ice for 10 min, cell debris was removed by centrifugation (15 min at 15 000×g). The supernatant was filter-sterilized by passage through a 0.2 μm filter and applied to a 6 ml globotriose-Sepharose affinity column59 equilibrated with 0.5 M NaCl in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4). The column was washed with 40 volumes of 0.5 M NaCl in PBS prior to eluting VT-1 with 6 M guanidine.HCl. Fractions of 1 ml were collected and immediately dialyzed against PBS to remove the denaturant. VT-1 B chain was isolated exactly the same way. The plasmid used here was pSBC32 which contains the coding sequence for the B chain in the high level expression vector pKK233-2 and was a kind gift of Dr SB Calderwood.60
Cell lines
Group I BL cell lines L3055 (EBVneg), BL2 (EBVneg), SAV (EBVpos), and Mutu I (EBVpos) were maintained in early passage as previously described.24,29 Late passage Mutu III cells were also included for study.29 Cells were maintained in continuous culture with RPMI 1640 supplemented with 10% pre-screened FCS (Bio-Whittacker, Wokingham, UK), 5000 IU/ml penicillin, 5 mg/ml streptomycin (Gibco/BRL, Paisley, UK) and 200 mM glutamine (Gibco/BRL). Stable bcl-2 transfectants were obtained as described previously.22,23 Stable bcl-xL transfectants were derived by electroporation of the plasmid vector pEF-MC1neopA61 containing the bcl-xL cDNA followed by selection of cells in G418 (Sigma, Poole, UK). Immunoblotting for Bcl-2 and Bcl-xL was carried out as detailed elsewhere.23,24 From the lines established, those showing high level expression of the genes transfected were chosen for study.
Selection of CD77neg cells
L3055 cells were cultured at a concentration of 4×105/ml for 48 h in the presence of VT-1 at 100 ng/ml. Dead cells were removed by centrifugation on Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). Remaining viable cells were collected from the interface, washed three times in culture medium and used as ‘CD77neg cells’ in the experiments described.
Flow cytometric analysis of CD77 expression
Cells were harvested after culture under the conditions indicated in the text. Cells were washed once in PBS with 0.1% BSA and 0.01% sodium azide (FACS buffer) before direct or indirect immunofluorescence analysis was performed. Briefly, cells were labeled by a 30 min incubation on ice with either directly-conjugated rat 38.13 mAb or unconjugated mAb that was detected by an additional second stage incubation with FITC-conjugated anti-rat IgM Ab (Serotec). In both cases, rat IgM anti-DNP was used as the control. Cells labeled either directly or indirectly were washed once and resuspended in FACS buffer. Flow cytometry data were obtained using an Epics-XL flow cytometer (Beckman Coulter, Miami, FL, USA). Viable cells were gated according to forward (FSC) and side (SSC) light scatter settings. Data were processed and analyzed using WinMDI software (Scripps Research Institute, La Jolla, CA, USA).
Measurement of DNA synthesis
DNA synthesis was determined by [3H]thymidine incorporation.31 After culture of 5×104 cells per 200 μl with additions as specified, wells were pulsed for the final 4 h with [3H]thymidine (Amersham Interanational, Amersham, UK, 10 μCi/ml in culture medium, 50 μl per well) and harvested on a Skatron cell harvester (Helis Bio Ltd, Newmarket, UK). All assays were performed in quadruplicate with replicates usually being within 10% and always within 15% of each other.
Cell viability and apoptosis assays
Cell viability was assessed by a standard trypan blue dye exclusion assay.31 Apoptosis was determined by staining treated cells with acridine orange and visualizing nuclear morphology exactly as described previously.22 Viable cells display a homogenous chromatin staining pattern whereas apoptotic cells show characteristically condensed and fragmented chromatin. Each determination was carried out on scoring 100 cells in duplicate.
Abbreviations
- BL:
-
Burkitt lymphoma
- CD40L:
-
CD40 ligand
- EBV:
-
Epstein-Barr virus
- GC:
-
germinal center
- TNF:
-
tumor necrosis factor
- VT:
-
Verotoxin
References
Jacewicz M, Clausen H, Nudelman E, Donohue-Rolfe A and Keus GT . (1986) Pathogenesis of Shigella diarrhea XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J. Exp. Med. 163: 1391–1404
Lindberg AA, Brown JE, Stromberg N, Westling-Ryd M, Schultz JE and Karlsson K-A . (1987) Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J. Biol. Chem. 262: 1779–1785
Lingwood CA, Law H, Richardson S, Petric M, Brunton JL, DeGrandis S and Karmali M . (1987) Glycolipid binding of purified and recombinant Escherichia coli-produced verotoxin in vitro. J. Biol. Chem. 262: 8834–8839
Waddell T, Cohen A and Lingwood CA . (1990) Induction of verotoxin sensitivity in receptor-deficient cell lines using the receptor glycolipid globotriaosylceramide. Proc. Natl. Acad. Sci. USA 87: 7898–7901
Lingwood CA . (1994) Verotoxin-binding in human renal sections. Nephron 66: 21–28
Simon M, Cleary TG, Hernandez JD and Abboud HE . (1998) Shiga toxin 1 elicits diverse biologic responses in mesangial cells. Kidney Int. 54: 1117–1127
Ramegowda B and Tesh VL . (1996) Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shia-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 64: 1173–1180
Bitzan M, Richardson S, Huang C, Boyd B, Petric M and Karamli MA . (1994) Evidence that verotoxins from Escherichia coli bind to P blood group antigens of human erythrocytes in vitro. Infect. Immun. 62: 3337–3347
Wiels J . (1998) CD77 workshop panel report, in Kishimoto T, et al (eds) Leucocyte Typing VI. New York, NY, Garland pp175–177
Hardie DL, Johnson GD, Khan M and MacLennan ICM . (1993) Quantitative analysis of molecules which distinguish functional compartments within germinal centers. Eur. J. Immunol. 23: 997–1004
MacLennan ICM . (1994) Germinal centers. Annu. Rev. Immunol. 12: 117–139
Wiels J, Fellous M and Tursz T . (1981) Monoclonal antibody against a Burkitt lymphoma associated antigen. Proc. Natl. Acad. Sci. USA 78: 6485–6489
Rooney CM, Gregory CD, Rowe M, Finerty S, Edwards C, Rupani H and Rickinson AB . (1986) Endemic Burkitt's lymphoma: phenotypic analysis of tumour biopsy cells and of dreived tumor cell lines. J. Natl. Cancer Inst. 77: 681–687
Gregory CD, Edwards CF, Milner A, Weils J, Lipinski M, Rowe M, Tursz T and Rickinson AB . (1988) Isolation of a normal B cell subset with a Burkitt-like phenotype and transformation in vitro with Epstein-Barr virus. Int. J. Cancer 42: 213–220
Sakthivel R, Christensson B, Ehlin-Henriksson B and Klein G . (1989) Immunophenotypic characterization of follicle-center-cell-derived non-Hodgkin's lymphomas. Int. J. Cancer 43: 624–630
Garred O, Dubinina E, Holm PK, Olsnes O, van Deurs B, Kozlov JV and Sandvig K . (1995) Role of processing and intracellular transport for optimal toxicity of Shiga toxin and toxin mutants. Exp. Cell Res. 218: 39–49
Johannes L and Goud B . (1998) Surfing on a retrograde wave: how does Shiga toxin reach the endoplasmic reticulum? Trends Cell. Biol. 8: 158–162
Mangeney M, Lingwood CA, Taga S, Caillou B, Tursz T and Wiels J . (1993) Apoptosis induced in Burkitt's lymphoma cells via Gb3/CD77, a glycolipid antigen. Cancer Research 53: 5314–5319
Taga S, Carlier K, Mishal Z, Capoulade C, Mangeney M, Lecluse Y, Couland D, Tetaud C, Pritchard LL, Tursz T and Wiels J . (1997) Intracellular signalling events in CD77-mediated apoptosis of Burkitt's lymphoma cells. Blood 90: 2757–2767
Liu YJ, Mason DY, Johnson GD, Abbot S, Gregory CD, Hardie DL, Gordon J and MacLennan ICM . (1991) Germinal center cells express bcl-2 protein after activation by signals which prevent their entry to apoptosis. Eur. J. Immunol. 21: 1905–1910
Holder MJ, Wang H, Milner AE, Casamjor M, Armitage R, Spriggs MK, Fanslow WC, MacLennan ICM, Gregory CD and Gordon J . (1993) Suppression of apoptosis in normal and neoplastic human B lymphocytes by CD40 ligand is independent of Bcl-2 induction. Eur. J. Immunol. 23: 2368–2371
Milner AE, Johnson GD and Gregory CD . (1992) Prevention of programmed cell death in Burkitt lymphoma cell lines by bcl-2-dependent and -independent mechanisms. Int. J. Cancer 52: 636–644
Wang H, Grand RJA, Milner AE, Armitage RJ, Gordon J and Gregory CD . (1996) Repression of apoptosis in human B-lymphoma cells by CD40-ligand and Bcl-2: relationship to the cell-cycle and role of the retinoblastoma protein. Oncogene 13: 373–379
Baker MP, Eliopoulos E, Young LS, Armitage RJ, Gregory CD and Gordon J . (1998) Prolonged phenotypic, functional, and molecular change in group I Burkitt lymphoma cells on short-term exposure to CD40 ligand. Blood 92: 2830–2843
O'Conor GT . (1961) Malignant lymphoma in African children. II. Pathological entity. Cancer 14: 270–283
Epstein MA and Herdson PB . (1963) Cellular degeneration associated with characteristic nuclear fine structure changes in the cells from two cases of Burkitt's malignant lymphoma syndrome. Brit. J. Cancer 17: 56–58
Berard C, O'Conor GT, Thomas LB and Torloni H . (1969) Histopathological definition of Burkitt's lymphoma. Bull. WHO 40: 601–607
Grafton G, Goodall M, Gregory CD and Gordon J . (1997) Mechanisms of antigen receptor-dependent apoptosis in human B lymphoma cell lines probed with a panel of 27 monoclonal antibodies. Cell. Immunol. 182: 45–56
Gregory CD, Dive C, Henderson S, Smith CA, Williams GT, Gordon J and Rickinson AB . (1991) Activation of Epstein-Barr virus latent genes protects human B cells from death by apoptosis. Nature 349: 612–614
Gregory CD and Milner AE . (1994) Regulation of cell survival in Burkitt lymphoma: implications from studies of apoptosis following cold-shock treatment. Int. J. Cancer 57: 419–426
MacDonald I, Wang H, Grand R, Armitage RJ, Fanslow WC, Gregory CD and Gordon J . (1996) Transforming growth factor-β1 cooperates with anti-immunoglobulin for the induction of apoptosis in group I (biopsy-like) Burkitt lymphoma cell lines. Blood 87: 1147–1154
Geser A, Lenoir GM, Anvret M, Bornkamm G, Klein G, Williams EH, Wright DH and De The G . (1983) Epstein-Barr virus markers in a series of Burkitt's lymphomas from the West Nile district of Uganda. Europ. J. Cancer Clin. Oncol. 19: 1393–1404
Gregory CD, Rowe M and Rickinson AB . (1990) Different Epstein-Barr virus B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J. Gen. Virol. 71: 1481–1495
Rowe M, Rowe DT, Gregory CD, Young LS, Farrel PJ, Rupani H and Rickinson AB . (1987) Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 6: 2743–2751
Ambinder FR and Griffin CA . (1991) Biology of the lymphomas: cytogenetics, molecular biology, and virology. Curr. Opin. Oncol. 3: 806–812
Veronese ML, Schichman SA and Croce CM . (1996) Molecular diagnosis of lymphoma. Curr. Opin. Oncol. 8: 346–352
Gupta RK and Lister TA . (1996) Current management of follicular lymphoma. Curr. Opin. Oncol. 8: 360–365
Zhang X, Li L, Choe J, Krajewski S, Reed JC, Thompson C and Choi YS . (1996) Up-regulation of Bcl-xL expression protects CD40-activated human B cells from Fas-mediated apoptosis. Cell. Immunol. 173: 149–154
Tuscano JM, Druey KM, Riva A, Pena J, Thompson CB and Kehrl JH . (1996) Bcl-x rather than Bcl-2 mediates CD40-dependent centrocyte survival in the germinal center. Blood 88: 1359–1364
Wang Z, Karras JG, Howard RG and Rothstein TL . (1995) Induction of bcl-x by CD40 engagement rescues sIg-induced apoptosis in murine B cells. J. Immunol. 155: 3722–3275
Choi MSK, Boise LH, Gottschalk AR, Quintans J, Thompson CB and Klaus GGB . (1995) The role of Bcl-xL in CD40-mediated rescue from anti-μ-induced apoptosis in WEHI-231 B lymphoma cells. Eur. J. Immunol. 25: 1352–1357
Foy TM, Aruffo A, Bojorath J, Buhlman JE and Noelle RJ . (1996) Immune regulation by CD40 and its ligand gp39. Annu. Rev. Immunol. 14: 591–617
Casamayor-Palleja M, Khan M and MacLennan ICM . (1995) A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J. Exp. Med. 181: 1293–1301
Grammer AC, Bergman MC, Miura Y, Fujita K, Davis LS and Lipsky PE . (1995) The CD40 ligand expressed by human B cells costimulates B cells responses. J. Immunol. 154: 4996–5010
Wykes M, Poudrier J, Lindstedt R and Gray D . (1998) Regulation of cytoplasmic, surface and soluble forms of CD40 ligand in mouse B cells. Eur. J. Immunol. 28: 548–559
Mangeney M, Richard Y, Coulaud D, Tursz T and Wiels J . (1991) CD77: an antigen of germinal center B cells entering apoptosis. Eur. J. Immunol. 21: 1131–1140
Ghia P, Boussiotis VA, Schultze JL, Cardoso AA, Dorfman DM, Gribben JG, Freedman AS and Nadler LM . (1998) Unbalanced expression of bcl-2 family proteins in follicular lymphoma: contribution of CD40 signaling in promoting survival. Blood 91: 244–251
McCloskey N, Pound JD, Holder MJ, Williams JM, Roberts LM, Lord JM and Gordon J . (1999) The extrafollicular-to-follicular transition of human B lymphocytes: induction of functional globotriaosylceramide (CD77) on high threshold occupancy of CD40. Eur. J. Immunol. 29: 3236–3244
Laherty CD, Hu HM, Opipari AW, Wang F, Dixit VM . (1992) Epstein-Barr virus LMP-1 gene product induces A20 zinc finger protein expression by activating nuclear factor kB. J. Biol. Chem. 267: 24157–24160
Opipari AW Jnr, Hu HM, Yabkowitz R and Dixit VM . (1992) The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J. Biol. Chem. 267: 12424–12427
Young LS, Eliopoulos AG, Gallagher NJ, Dawson CW . (1998) CD40 and epithelial cells: across the great divide. Immunol. Today 19: 502–506
Michael JM, Lavin MF and Watters DJ . (1997) Resistance to radiation-induced apoptosis in Burkitt's lymphoma cells is associated with defective ceramide signaling. Cancer Res. 57: 3600–3605
Keppler-Hafkemeyer A, Brinkman U and Pastan I . (1998) Role of caspases in immunotoxin-induced apoptosis of cancer cells. Biochemistry 37: 16934–16942
Mahan JD, McAllister C and Chiasera J . (1997) Inhibition of caspases decreases verocytotoxin-1 (VT-1) mediated apoptosis but not cell death in vero cells. J. Am. Soc. Nephrol. 8: (S) A2331
Besser RE, Griffen PM and Slutsker L . (1999) Escherichia coli O157:H7 gastroenteritis and the hemolytic uremic syndrome: an emerging infectious disease. Annu. Rev. Med. 50: 355–367
Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS and Lior H . (1985) The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151: 775–782
White CA, Larocca A and Grillo-Lopez AJ . (1999) Anti-CD20 monoclonal antibodies as novel treatments for non-Hodgkin's lymphoma. Pharm. Sci. Technol. Today 2: 95–101
Morris AE, Remmele R, Klinke R, MacDuff BM, Whitney L and Armitage RJ . (1999) Incorporation of an isoleucine zipper motif enhances the activity of soluble CD40L. J. Biol. Chem. 274: 418–423
Müller D, Vic G, Critchley P, Crout DGH, Lea N, Roberts LM and Lord JM . (1998) Chemical synthesis of globotriose and galabiose: relative stabilities of their complexes with Escherichia coli Shiga-like toxin-1 as determined by denaturation-titration with guanidinium hydrochloride. J. Chem. Soc. Perkin Trans. 1: 2287–2294
Calderwood SB, Acheson DWK, Goldberg MB, Boyko SA and Donohoue-Rolfe A . (1990) A system for production and rapid purification of large amounts of the Shia toxin/Shiga-like toxin I B subunit. Inf. Imm. 58: 2977–2982
Visvader JE, Elefanty AG, Strasser A and Adams JM . (1992) GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO J. 11: 4557–4564
Acknowledgements
Work at Birmingham was supported by a Programme Grant from the Medical Research Council (UK), at Nottingham by the Leukaemia Research Fund, and at Warwick by the Wellcome Trust (Grant 050856/Z/97/Z). J Gordon is a MRC Non-Clinical Professor.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Nunez
Rights and permissions
About this article
Cite this article
Gordon, J., Challa, A., Levens, J. et al. CD40 ligand, Bcl-2, and Bcl-xL spare group I Burkitt lymphoma cells from CD77-directed killing via Verotoxin-1 B chain but fail to protect against the holotoxin. Cell Death Differ 7, 785–794 (2000). https://doi.org/10.1038/sj.cdd.4400710
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400710
Keywords
This article is cited by
-
Biochemical, pathological and oncological relevance of Gb3Cer receptor
Medical Oncology (2011)
-
TGF-β induces apoptosis in human B cells by transcriptional regulation of BIK and BCL-XL
Cell Death & Differentiation (2009)
-
Study on induction of apoptosis on HeLa and Vero cells by recombinant shiga toxin and its subunits
Cytotechnology (2009)
-
Retrograde transport pathways utilised by viruses and protein toxins
Virology Journal (2006)
-
Fibrates and medroxyprogesterone acetate induce apoptosis of primary Burkitt's lymphoma cells and cell lines: potential for applying old drugs to a new disease
Leukemia (2003)