Abstract
Bcl-2 and its relative, Bcl-xL, inhibit apoptotic cell death primarily by controlling the activation of caspase proteases. Previous reports have suggested at least two distinct mechanisms: Bcl-2 and Bcl-xL may inhibit either the formation of the cytochrome c/Apaf-1/caspase-9 apoptosome complex (by preventing cytochrome c release from mitochondria) or the function of this apoptosome (through a direct interaction of Bcl-2 or Bcl-xL with Apaf-1). To evaluate this latter possibility, we added recombinant Bcl-xL protein to cell-free apoptotic systems derived from Jurkat cells and Xenopus eggs. At low concentrations (50 nM), Bcl-xL was able to block the release of cytochrome c from mitochondria. However, although Bcl-xL did associate with Apaf-1, it was unable to inhibit caspase activation induced by the addition of cytochrome c, even at much higher concentrations (1–5 μM). These observations, together with previous results obtained with Bcl-2, argue that Bcl-xL and Bcl-2 cannot block the apoptosome-mediated activation of caspase-9. Cell Death and Differentiation (2000) 7, 402–407
Similar content being viewed by others
Introduction
The process of apoptotic cell death is orchestrated through the activation of a family of cysteine proteases, the caspases (reviewed, e.g. by1,2). These enzymes cleave and activate certain key substrates that produce the characteristic morphological and biochemical events of apoptosis, e.g., cell shrinkage, membrane blebbing, phosphatidylserine externalization, chromatin condensation, and DNA fragmentation, thereby ensuring the orderly disposal of the dead cell. Apoptosis is regulated by proteins belonging to the Bcl-2 family (reviewed, e.g. in3,4), which can be either pro- (e.g., Bax, Bak) or anti-apoptotic (e.g., Bcl-2, Bcl-x). Therefore, one of the keys to understanding apoptosis is to determine how the Bcl-2 family members control caspase activation.
One of the major ways in which apoptosis proceeds is via the mitochondria, which release cytochrome c from the intermembrane space following an apoptotic signaling event.5,6 In the cytosol, holocytochrome c binds to Apaf-1, which then oligomerizes, recruiting procaspase-9 into the complex, which is called an ‘apoptosome’.7,8,9 This activates the procaspase, which can now cleave substrates, including other procaspases (specifically, procaspases-3 and -7), activating them in turn. These activated ‘executioner’ caspases proceed to cleave key substrates in the cell, including ICAD/DFF45, which upon cleavage releases an active nuclease (CAD/DFF40). CAD/DFF40 is then transported into the nucleus, where it cleaves the DNA into oligonucleosomal fragments.10,11,12 Other identified caspase substrates, when cleaved, mediate membrane blebbing and other features of apoptosis.
An important way in which anti-apoptotic Bcl-2 family members prevent apoptosis is to prevent the release of cytochrome c (which initiates the cascade described above). Bcl-2 and Bcl-xL bind to the outer mitochondrial membrane and block cytochrome c release both in vitro and in vivo.5,6 This inhibition may involve two nonexclusive mechanisms: (a) inactivation of pro-apoptotic Bcl-2 family members (e.g., Bax, Bak) which themselves induce the release of cytochrome c, or (b) direct interference with the specific (and as yet controversial) mechanism of cytochrome c release, perhaps via the formation of ion channels. Because the release of cytochrome c is blocked, the cell does not activate caspases and apoptosis is prevented; in addition, mitochondrial functions, such as respiration, remain intact and the cell remains viable.
Recently, alternative functions of anti-apoptotic Bcl-2 family members have been proposed, some of which are suggested to act downstream of the mitochondria, at the level of the apoptosome. In the nematode, C. elegans, the anti-apoptotic Bcl-2 family member Ced-9 binds to and inactivates Ced-4, which otherwise functions to bind and activate a procaspase, Ced-3, leading to cell death (reviewed in13,14). By analogy, the mammalian Ced-4 homolog Apaf-1 could be similarly neutralized by an anti-apoptotic Bcl-2 family member. One recent report has suggested that this is the case; co-expressed Bcl-xL and Apaf-1 associate,15,16 and this appears to inhibit apoptosis induced by co-expression of Apaf-1 and procaspase-9.15 Further support for anti-apoptotic Bcl-2 family members acting downstream of the mitochondria include an apparent ability of Bcl-xL and Bcl-2 to block apoptosis induced by microinjection of cytochrome c17,18 and similarly an apparent ability of Bcl-2 to inhibit Bax-induced apoptosis but not Bax-induced cytochrome c release in transiently transfected cells.19 Another recent report found that high concentrations of Bcl-2 or Bcl-xL, added to a cell-free system, effectively prevented caspase activation induced by the addition of cytochrome c.20
Together, these observations suggest that anti-apoptotic Bcl-2 family members can directly interfere with the formation or function of the vertebrate apoptosome (cytochrome c/Apaf-1/procaspase-9). This is in contrast with one report showing that Bcl-xL was unable to block death induced by microinjection of cytochrome c21 as well as with our earlier studies showing that Bcl-2 does not interfere with cytochrome c-induced caspase activation in a cell free system (but does inhibit cytochrome c release from mitochondria.5 Since Bcl-xL is suggested to bind directly to Apaf-1, it was therefore important to determine whether Bcl-xL can interfere with the ability of cytochrome c to activate caspases.
Results and Discussion
We employed both mammalian and Xenopus cell-free systems to evaluate the effects of a recombinant Bcl-xL protein on mitochondrial release of cytochrome c and caspase activation. We first determined the concentrations of Bcl-xL that are effective in preventing apoptosis in cells. Jurkat cells stably transfected with Bcl-xL were relatively resistant to apoptosis (not shown), and the amount of Bcl-xL present was compared to known amounts of recombinant protein. In this manner, we estimated that the concentration of Bcl-xL in Jurkat cells overexpressing the protein is ≈15–30 nM.
We then determined the efficacy of the recombinant Bcl-xL protein in vitro, using a system we described previously.22 Cytosolic extracts from Jurkat cells were mixed with isolated mouse liver mitochondria in the presence or absence of 200 nM Bcl-xL, and then treated with caspase-8. In the experiment shown in Figure 1, the caspase-8-activated cytosol induced the release of cytochrome c from the mitochondria, as previously observed. This is likely to be an effect of the cleavage of Bid by caspase-8, which activates Bid to bind to mitochondria and trigger cytochrome c release.23,24 Previous results showed that as little as 40 nM Bcl-xL was sufficient for complete inhibition of cytochrome c release in this assay.22 The requirement in the cell-free system for a concentration of recombinant Bcl-xL (40 nM) that is somewhat higher than that seen in Bcl-xL-overexpressing Jurkat cells (≈15–30 nM) may reflect a reduced activity of the bacterially expressed protein due to misfolding, lack of postsynthetic modifications, or the lack of the C-terminal membrane insertion domain.
Bcl-xL inhibits release of cytochrome c from mitochondria in a cell-free system. Jurkat cell cytosol was mixed with mouse liver mitochondria either in the absence (top) or presence (bottom) of recombinant Bcl-xL (200 nM). Extracts were activated by the addition of recombinant caspase-8 (100 nM); then, at the indicated times, mitochondria were removed by centrifugation and the supernatants evaluated for the presence of cytochrome c by immunoblotting. MF: cytochrome c content of control mitochondrial fraction
In contrast, Bcl-xL protein failed to inhibit the ability of cytochrome c plus dATP to trigger the activation of caspases in cytosolic extracts, an effect that is dependent upon Apaf-1 and procaspase-9.8 In the experiment shown in Figure 2A, Bcl-xL at concentrations up to 10 μM failed to inhibit the generation of DEVDase activity upon addition of cytochrome c and dATP. Examination of the relevant caspases in this experiment (Figure 2B) revealed a marginal effect on the processing of procaspase-9 at the highest concentration of Bcl-xL, and this was mirrored in a slight reduction in procaspase 3 processing. However, this effect was only seen at concentrations that were 250 times the dose of Bcl-xL needed to inhibit cytochrome c release from mitochondria, and over 300 times that found in Jurkat cells overexpressing the Bcl-xL gene.
Bcl-xL does not inhibit caspase activation induced by the addition of cytochrome c and dATP to Jurkat cell extracts. (A) dATP (1 mM) and cytochrome c (10 μM) were added to Jurkat cell cytosol containing Bcl-xL at the indicated concentrations; after 1 h, samples were taken for measurement of initial rates of DEVDase activity. Note that Bcl-xL, even at concentrations as high as 10 μM, did not inhibit DEVDase activation. (B) In the same experiment, immunoblot analysis was used to detect the processing of Caspases-9 and -3. Note that Bcl-xL had either no effect (concentrations of 5 μM or below) or only a slight inhibitory effect (at 10 μM) on caspase activation
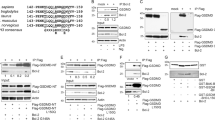
The inability of the recombinant Bcl-xL to block Apaf-1 activity (while potently inhibiting mitochondrial release of cytochrome c) might have been due to a failure of this recombinant protein to bind to Apaf-1 (the native protein reportedly binds to Apaf-1 when both are co-expressed and immunoprecipitated).15,16 Therefore, we expressed FLAG-tagged full length or truncated Apaf-1 (530), containing only the CARD and Ced-4 domains,25 in 293T cells by transient transfection. Lysates from these cells were incubated with recombinant Bcl-xL protein. As shown in Figure 3, anti-FLAG antibody effectively precipitated the Apaf-1 and recombinant Bcl-xL proteins. The truncated form of Apaf-1 displayed reduced binding to Bcl-xL.
Bcl-xL binds in vitro to Apaf-1. 293T cells were transiently transfected with cDNAs coding for Flag-tagged wild-type Apaf-1 (WT) or a truncated form of Apaf-1 (530) corresponding to amino acid residues 1–530. Lysates were incubated with anti-Flag antibody immobilized on Protein G-Sepharose beads. The beads were then incubated in a solution containing recombinant Bcl-xL, washed, and loaded on an SDS-polyacrylamide gel. Shown are immunoblots of the bound material probed with anti-Apaf-1 (top) and anti-Bcl-xL (bottom) antibodies
It is important to note that while our results demonstrate that Apaf-1 and Bcl-xL can bind under these artificial conditions, this cannot be interpreted as saying that these proteins physically interact under physiological conditions. Indeed, our results in Figure 2 support the idea that even when Bcl-xL DOES bind to Apaf-1, the interaction does not influence Apaf-1 function, i.e., cytochrome c-triggered activation of procaspase-9.
One of the first systems in which a role for mitochondria in apoptosis was demonstrated involved the use of Xenopus egg extracts, in which nuclear condensation, DNA fragmentation and caspase activation proceed spontaneously, but only in the presence of the mitochondrial fraction.5,26,27 Using this system, we previously demonstrated that after a few hours of incubation, the mitochondria in these extracts spontaneously release cytochrome c, which in turn triggers caspase activation. Bcl-2 functions to block the release of cytochrome c but does not interfere with the effects of exogenously added cytochrome c.5
We therefore extended these observations using recombinant Bcl-xL. In the experiment shown in Figure 4A, Xenopus egg cytosol was mixed with Xenopus egg mitochondria and incubated in either the presence or absence of recombinant Bcl-xL. At various times, the mitochondrial content of cytochrome c was measured by immunoblotting, and caspase activity was measured as the rate of cleavage of z-DEVD-pNA. As shown in Figure 4A (left panel), when incubations were carried out at 22°C in the absence of Bcl-xL, mitochondria were completely depleted of cytochrome c between 2 and 4 h of incubation. Reducing the temperature to 0°C blocked cytochrome c release, as previously observed.5,27 Figure 4A (right panel) shows that DEVDase activity became apparent at approximately 3.5 h of incubation. (In this system caspase activation was previously shown to depend on cytochrome c release from mitochondria).5,27 However, the addition of as little as 50 nM Bcl-xL caused a substantial inhibition of cytochrome c release from mitochondria (left panel) and of caspase (DEVDase) activation (right panel).
In Xenopus extracts, Bcl-xL blocks cytochrome c release but not cytochrome c-mediated activation of caspases. (A) Bcl-xL blocks mitochondrial cytochrome c release and DEVDase activation. Extracts containing Xenopus egg cytosol and mitochondria (2 mg/ml) were incubated at either 0°C or 22°C in the presence or absence of Bcl-xL. At the times indicated, aliquots were assessed for cytochrome c translocation (left) and DEVDase activation (right; 22°C only). (B) Bcl-xL (1 μM) did not reverse the effects of cytochrome c (any concentration). Horse heart cytochrome c (HHCc) was added, in the presence or absence of Bcl-xL, to a simplified extract mix of cytosol and mitochondria (left; see A) or cytosol only (right). DEVD-specific protease activity measured at 30 min showed Bcl-xL to have no inhibitory effect at any concentration of HHCc. Similar results were obtained at 15 and 60 min (not shown). Bcl-xL (1 μM) was present at 20 times the concentration capable of blocking mitochondria-dependent DEVDase activation (A). The data are presented as mean and standard deviation of triplicate incubations. The experiment is representative of two independent experiments
In contrast, when cytochrome c was added to the extracts, DEVDase activity was induced within 30 min, but there was no effect at any concentration of Bcl-xL (up to 1 μM) on this cytochrome c-induced effect (Figure 4B). This was true both in the presence and absence of mitochondria. Thus, while Bcl-xL blocked the activation of caspases in this system at the level of the mitochondria, addition of cytochrome c completely bypassed the inhibition by Bcl-xL. These results are in agreement with our former observations with Bcl-25 and with the effects of Bcl-xL in mammalian extracts, above.
The ability of Bcl-xL to block caspase activation during apoptosis is therefore best explained by its ability to interfere with mitochondrial release of cytochrome c, and not by inhibition at some downstream site, such as the function of Apaf-1. This is in contrast to the apparent ability of C. elegans Ced-9 to interfere directly with the caspase-activating function of Ced-4.28,29,30 The observation that Bcl-xL and Apaf-1 can be co-immunoprecipitated in vitro may only be relevant to situations where large amounts of these proteins are present, as when the proteins are overexpressed in cells. Supporting this, Moriishi et al. recently reported that Bcl-2 family members do not directly interact with Apaf-1.31
However, it remains possible that Bcl-xL and other anti-apoptotic Bcl-2 family members bind to and inactivate other unidentified Ced-4 homolog in vertebrate cells; indeed, the ability of cells lacking Apaf-1 to undergo apoptosis (albeit with lower sensitivity) suggests that such homologs exist. However, the severe developmental defects seen in Apaf-1 null mice, as well as the dramatically reduced susceptibility to apoptosis in cells from these animals,32,33 indicates that Apaf-1 and its regulators are major players in the apoptotic process.
How do anti-apoptotic Bcl-2 family members block cytochrome c release? There are at least two nonexclusive possibilities. First, the members of this family have a propensity to bind to each other via Bcl-2 homology (BH) domains, especially BH3, and thus can influence the activity of other family members (pro- or anti-apoptotic). Secondly, Bcl-xL and other members of this family are structurally related to bacterial pore-forming proteins, and are known to insert into intracellular membranes, including the mitochondrial outer membrane; thus, they may control apoptosis at least partly by acting as ion channels.34,35,36,37,38 If so, then there are two alternate scenarios, depending on whether these channels directly induce or inhibit the mechanism responsible for cytochrome c release. In the former case, the anti-apoptotic Bcl-2 family members would bind and neutralize the activity of the pro-apoptotic family members, which actively induce cytochrome c release (thus, in this model the pro-apoptotic family members are active, and the anti-apoptotic members repress their activity).39 Alternatively, the anti-apoptotic Bcl-2 family members could directly act to interfere with the primary mechanism of cytochrome c release, and this activity would be repressed by the pro-apoptotic Bcl-2 members.40 Currently, the nature of the mechanism responsible for the release of cytochrome c remains unclear, and therefore either model is equally attractive.
Materials and Methods
Recombinant Bcl-xL was prepared as described.22 Anti-Flag antibody was obtained from Sigma; monoclonal anti-cytochrome c (7H8.2C12, 65981A), polyclonal anti-caspase-3 (65906E), and polyclonal anti-Bcl-xL (65181E) were from Pharmingen, San Diego, CA, USA. Polyclonal antibodies to caspase-9 and Apaf-1 were raised in rabbits against recombinant caspase-9 and a synthetic Apaf-1 peptide (S38EEEKVRNEPTQQQR52), respectively.41
Co-immunoprecipitation of Apaf-1 and Bcl-xL
Using the calcium phosphate method, 293T cells were transiently transfected with N-terminally Flag-tagged cDNAs corresponding to Apaf-1 (wild-type) or Apaf-1 (530), a truncated form consisting of amino acids 1-530. After 24 h, cells were harvested and washed twice with ice-cold PBS. Cell pellets were lysed by incubating 30 min on ice in buffer A: 50 mM HEPES, pH 7.4, 1% NP-40, 150 mM NaCl, 10% glycerol, 1 mM EDTA, 2 mM dithiothreitol, and protease inhibitors. The lysates were microcentrifuged at 15 000×g for 10 min. Protein G-Sepharose was pre-incubated for 3 h at 4°C with 5 μl M2 α-Flag antibody and washed three times with buffer A. The beads were then incubated with extract samples for 3 h at room temperature and washed three times in buffer A. The beads were then resuspended in 200 μl of buffer A containing 5 μg of recombinant Bcl-xL. After a 3 h incubation at room temperature, the beads were washed three times in buffer A and resuspended in SDS–PAGE sample buffer. Immunoblots were performed as described.42 Anti-caspase-9 polyclonal rabbit serum was diluted 1 : 1500; anti-caspase-3 antibodies 1 : 2000.
Mammalian cell-free assays
Jurkat cell extracts (final protein concentration 12 mg/ml) were prepared as previously described.43 Cell-free apoptosis assays using isolated mitochondria were performed as recently described.22 Briefly, 50 μl of Jurkat cytosol (50 μg protein) containing mitochondria (3 μg), with or without recombinant Bcl-xL (200 nM), was incubated at 37°C with 100 nM recombinant active caspase-8. At various times, aliquots were taken and mitochondria re-isolated by microcentrifugation for 10 min at 15 000 r.p.m. In other experiments, 10 μM cytochrome c and 1 mM dATP were added to cytosol.
DEVDase assays in Jurkat cell extracts were performed as described,41 using DEVD-AFC (Enzyme Systems Products) to detect caspase-3-like activity. Briefly, cytosol samples (40 μg) were incubated with DEVD-AFC in caspase buffer [20 mM PIPES, 100 mM NaCl, 10 mM dithiothreitol, 1 mM EDTA, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonic acid (CHAPS), 10% sucrose, pH 7.2] at 37°C. Initial rates of substrate hydrolysis were determined using a Tecan SpectroFluor fluorimeter in the kinetic mode. Excitation was at 400 nm and emission was at 500 nm (slit widths 10 nm). A standard curve of AFC concentration versus fluorescence was used to determine the concentration of AFC generated from substrate hydrolysis.
Xenopus egg extracts
Xenopus eggs were fractionated as described previously.5,26,27,44 Extracts were reconstituted using cytosol (with ATP regenerating system: 14 mM phosphocreatine, 7 mM ATP, 150 mg/ml creatine phosphokinase) and heavy membranes (HM) which are enriched in mitochondria. DEVD-specific protease activity was measured by incubating 2-μl extract samples with z-DEVD-pNA (40 μM, Enzyme Systems Products, CA) in 200 μ of ELB buffer (250 mM sucrose, 20 mM HEPES/KOH, pH 7.5, 50 mM KCl, 2.5 mM MgCl2, 1 mM DTT). The initial increase in A405 over 30 min was used as a measure of the DEVDase activity. Cytochrome c redistribution from mitochondria to cytosol was analyzed by immunoblot5 of the mitochondrial pellet derived from 10 μl of extract.
Abbreviations
- HHCc:
-
horse heart cytochrome c
- HEPES:
-
N[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]
- EDTA:
-
ethylenediaminetetraacetic acid
- PIPES:
-
piperazine-N,N′-bis[2-ethanesulfonic acid]
- DEVDase:
-
caspase activity causing cleavage of Asp-Glu-Val-Asp
- DEVD-AFC:
-
N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin
- z-DEVD-pNA:
-
carbobenzoxy-Asp-Glu-Val-p-nitroanilide
References
Thornberry NA and Lazebnik Y . (1998) Caspases: enemies within. Science 281: 1312–1316
Villa P, Kaufmann SH and Earnshaw WC . (1997) Caspases and caspase inhibitors. Trends. Biochem. Sci. 22: 388–393
Reed JC . (1998) Bcl-2 family proteins. Oncogene 17: 3225–3236
Adams JM and Cory S . (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326
Kluck RM, Bossy-Wetzel E, Green DR and Newmeyer DD . (1997) The release of cytochromec from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP and Wang X . (1997) Prevention of apoptosis by Bcl-2: release of cytochromec from mitochondria blocked. Science 275: 1129–1132
Zou H, Li Y, Liu X and Wang X . (1999) An APAF-1. Cytochromec multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274: 11549–11556
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES and Wang X . (1997) Cytochromec and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489
Zou H, Henzel WJ, Liu X, Lutschg A and Wang X . (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochromec-dependent activation of caspase-3. Cell 90: 405–413
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A and Nagata S . (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50
Sakahira H, Enari M and Nagata S . (1998) Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391: 96–99
Liu X, Zou H, Slaughter C and Wang X . (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184
Horvitz HR . (1999) Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 59: 1701s–1706s
Metzstein MM, Stanfield GM and Horvitz HR . (1998) Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 14: 410–416
Hu Y, Benedict MA, Wu D, Inohara N and Nunez G . (1998) Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc. Natl. Acad. Sci. USA. 95: 4386–4391
Pan G, O'Rourke K and Dixit VM . (1998) Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex. J. Biol. Chem. 273: 5841–5845
Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ and Fritz LC . (1997) Cell-specific induction of apoptosis by microinjection of cytochromec. Bcl-xL has activity independent of cytochromec release. J. Biol. Chem. 272: 30299–30305
Brustugun OT, Fladmark KE, So DS, Orrenius S and Zhivotovsky B . (1998) Apoptosis induced by microinjection of cytochromec is caspase-dependent and is inhibited by Bcl-2. Cell Death Differ. 5: 660–668
Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B and Borner C . (1998) Bcl-2 prolongs cell survival after Bax-induced release of cytochromec. Nature 391: 496–499
Cosulich SC, Savory PJ and Clarke PR . (1999) Bcl-2 regulates amplification of caspase activation by cytochromec. Curr. Biol. 9: 147–150
Duckett CS, Li F, Wang Y, Tomaselli KJ, Thompson CB and Armstrong RC . (1998) Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochromec. Mol. Cell. Biol. 18: 608–615
Bossy-Wetzel E and Green DR . (1999) Caspases induce cytochromec release from mitochondria by activating cytosolic factors. J. Biol. Chem. 274: In press.
Li H, Zhu H, Xu CJ and Yuan J . (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501
Luo X, Budihardjo I, Zou H, Slaughter C and Wang X . (1998) Bid, a Bcl2 interacting protein, mediates cytochromec release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490
Srinivasula SM, Ahmad M, Fernandes-Alnemri T and Alnemri ES . (1998) Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell. 1: 949–957
Newmeyer DD, Farschon DM and Reed JC . (1994) Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirements for an organelle fraction enriched in mitochondria. Cell 79: 353–364
Kluck RM, Martin SJ, Hoffman BM, Zhou JS, Green DR and Newmeyer DD . (1997) Cytochromec activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 16: 4639–4649
James C, Gschmeissner S, Fraser A and Evan GI . (1997) CED-4 induces chromatin condensation in Schizosaccharomyces pombe and is inhibited by direct physical association with CED-9. Curr. Biol. 7: 246–252
Wu D, Wallen HD, Inohara N and Nunez G . (1997) Interaction and regulation of the Caenorhabditis elegans death protease CED-3 by CED-4 and CED-9. J. Biol. Chem. 272: 21449–21454
Chinnaiyan AM, O'Rourke K, Lane BR and Dixit VM . (1997) Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science 275: 1122–1126
Moriishi K, Huang DC, Cory S and Adams JM . (1999) Bcl-2 family members do not inhibit apoptosis by binding the caspase activator Apaf-1. Proc. Natl. Acad. Sci. USA. 96: 9683–9688
Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA and Gruss P . (1998) Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94: 727–737
Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM and Mak TW . (1998) Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94: 739–750
Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R and Martinou JC . (1997) Inhibition of Bax Channel-Forming Activity by Bcl-2. Science 277: 370–372
Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M and Reed JC . (1997) Channel formation by antiapoptotic protein Bcl-2. Proc. Natl. Acad. Sci. USA. 94: 5113–5118
Schendel SL, Montal M and Reed JC . (1998) Bcl-2 family proteins as ion-channels. Cell Death Differ. 5: 372–380
Matsuyama S, Schendel SL, Xie Z and Reed JC . (1998) Cytoprotection by Bcl-2 requires the pore-forming alpha5 and alpha6 helices. J. Biol. Chem. 273: 30995–31001
Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G and Korsmeyer SJ . (1997) Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc. Natl. Acad. Sci. USA. 94: 11357–11362
Oltvai ZN and Korsmeyer SJ . (1994) Checkpoints of dueling dimers foil death wishes. Cell 79: 189–192
Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M and Thompson CB . (1997) Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature 385: 353–357
Wolf BB, Goldstein JC, Stennicke HR, Beere H, Amarante-Mendes GP, Salvesen GS and Green DR . (1999) Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood (In press)
Bossy-Wetzel E, Newmeyer DD and Green DR . (1998) Mitochondrial cytochromec release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 17: 37–49
Martin SJ, Newmeyer DD, Mathias S, Farschon DM, Wang H-G, Reed JC, Kolesnick RN and Green DR . (1995) Cell-free reconstitution of Fas-, UV radiation- and ceramide-induced apoptosis. EMBO J. 14: 5191–5200
Newmeyer DD . (1998) Analysis of apoptotic events using a cell-free system based on Xenopus laevis eggs. In: Herzenberg LH and LA Blackwell C and Weir DM (eds). Weir's Handbook of Experimental Immunology, Vol.III. Blackwell Scientific. pp. 127.1–127.6
Acknowledgements
We thank Drs FS Alnemri and SM Srinivasula for the Flag CMV2 Apaf-1 (wild-type) and Apaf-1 (530) constructs; C Thompson and A Minn for the pET29bΔC construct, and G Salvesen for the generous gift of recombinant caspase-8. This work was supported by National Institute of Health grants to DR Green (CA69381, AI40646) and DDN (GM50284) and by a fellowship to E Bossy-Wetzel from the Swiss National Science Foundation (823A-046638). This is La Jolla Institute for Allergy and Immunology Publication number 305.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by BA Osborne
Rights and permissions
About this article
Cite this article
Newmeyer, D., Bossy-Wetzel, E., Kluck, R. et al. Bcl-xL does not inhibit the function of Apaf-1. Cell Death Differ 7, 402–407 (2000). https://doi.org/10.1038/sj.cdd.4400665
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400665
Keywords
This article is cited by
-
Apoptosis in post-streptococcal glomerulonephritis and mechanisms for failed of inflammation resolution
Pediatric Nephrology (2023)
-
An in vitro cytotoxicity of glufosfamide in HepG2 cells relative to its nonconjugated counterpart
Journal of the Egyptian National Cancer Institute (2021)
-
Downregulation of GLYR1 contributes to microsatellite instability colorectal cancer by targeting p21 via the p38MAPK and PI3K/AKT pathways
Journal of Experimental & Clinical Cancer Research (2020)
-
Intracellular apoptotic pathways: a potential target for reducing joint damage in rheumatoid arthritis
Inflammation Research (2018)
-
In vitro evaluation of anticancer and antibacterial activities of cobalt oxide nanoparticles
JBIC Journal of Biological Inorganic Chemistry (2015)