Abstract
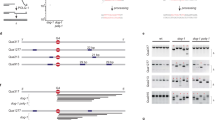
The repair of DNA double-strand breaks is essential for cells to maintain their genomic integrity. Two major mechanisms are responsible for repairing these breaks in mammalian cells, non-homologous end-joining (NHEJ) and homologous recombination (HR)1,2: the importance of the former in mammalian cells is well established3, whereas the role of the latter is just emerging. Homologous recombination is presumably promoted by an evolutionarily conserved group of genes termed the Rad52 epistasis group4,5,6,7,8,9,10,11. An essential component of the HR pathway is the strand-exchange protein, known as RecA in bacteria8 or Rad51 in yeast6. Several mammalian genes have been implicated in repair by homologous recombination on the basis of their sequence homology to yeast Rad51 (ref. 11): one of these is human XRCC2 (refs 12, 13). Here we show that XRCC2 is essential for the efficient repair of DNA double-strand breaks by homologous recombination between sister chromatids. We find that hamster cells deficient in XRCC2 show more than a 100-fold decrease in HR induced by double-strand breaks compared with the parental cell line. This defect is corrected to almost wild-type levels by transient transfection with a plasmid expressing XRCC2. The repair defect in XRCC2 mutant cells appears to be restricted to recombinational repair because NHEJ is normal. We conclude that XRCC2 is involved in the repair of DNA double-strand breaks by homologous recombination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rouet,P., Smith,F. & Jasin,M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol. Cell. Biol. 14, 8096–8106 (1994).
Liang,F., Han,M., Romanienko,P. J. & Jasin,M. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc. Natl Acad. Sci. USA 95, 5172–5177 (1998).
Jeggo,P. A. DNA breakage and repair. Adv. Gent. 38, 185–218 (1998).
Bezzubova,O., Silbergleit,A., Yamaguchi-Iwai,Y., Takeda,S. & Buerstedde,J. M. Reduced X-ray resistance and homologous recombination frequencies in a RAD54-/- mutant of the chicken DT40 cell line. Cell 89, 185–193 (1997).
Essers,J. et al. Disruption of mouse RAD54 reduces ionizing radiation resistance and homologous recombination. Cell 89, 195–204 (1997).
Nickoloff,J. A. & Hoekstra,M. F. in DNA Damage and Repair Vol. I (eds Nickoloff, J. A. & Hoekstra, M. F.) 335–362 (Humana, Totowa, 1998).
Rijkers,T. et al. Targeted inactivation of mouse RAD52 reduces homologous recombination but not resistance to ionizing radiation. Mol. Cell. Biol. 18, 6423–6429 (1998).
Smith,G. R. in DNA Damage and Repair Vol. I (eds Nickoloff, J. A. & Hoekstra, M. F.) 135–162 (Humana, Totowa, 1998).
Sonoda,E. et al. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 17, 598–608 (1998).
Yamaguchi-Iwai,Y. et al. Homologous recombination, but not DNA repair, is reduced in vertebrate cells deficient in RAD52. Mol. Cell. Biol. 18, 6430–6435 (1998).
Thacker,J. A surfeit of RAD51-like genes? Trends Genet. 15, 166–168 (1999).
Cartwright,R., Tambini,C. E., Simpson,P. J. & Thacker,J. The XRCC2 DNA repair gene from human and mouse encodes a novel member of the recA/RAD51 family. Nucleic Acids Res. 26, 3084–3089 (1998).
Liu,N. et al. XRCC2 and XRCC3, new human Rad51-family members, promote chromosome stability and protect against DNA cross-links and other damages. Molecular Cell 1, 783–793 (1998).
Jones,N. J., Cox,R. & Thacker,J. Isolation and cross-sensitivity of X-ray-sensitive mutants of V79-4 hamster cells. Mutat. Res. 183, 279–286 (1987).
Liang,F., Romanienko,P. J., Weaver,D. T., Jeggo,P. A. & Jasin,M. Chromosomal double-strand break repair in Ku80 deficient cells. Proc. Natl Acad. Sci. USA 93, 8929–8933 (1996).
Smih,F., Rouet,P., Romanienko,P. J. & Jasin,M. Double-strand breaks at the target locus stimulate gene targeting in embryonic stem cells. Nucleic Acids Res. 23, 5012–5019 (1995).
Jasin,M. Double-strand break repair and homologous recombination in mammalian cells. in DNA Damage and Repair Vol. III (eds Nickoloff, J. A. & Hoekstra, M. F.) (Humana, Totowa, in the press).
Cheong,N., Wang,X., Wang,Y. & Iliakis,G. Loss of S-phase-dependent radioresistance in irs-1 cells exposed to X-rays. Mutat. Res. 314, 77–85 (1994).
Tsuzuki,T. et al. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl Acad. Sci. USA 93, 6236–6340 (1996).
Lim, D.-S. & Hasty,P. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol. Cell. Biol. 16, 7133–7143 (1996).
Baumann,P., Benson,F. E. & West,S. C. Human Rad51 protein promotes ATP dependent homologous pairing and strand transfer reactions in vitro. Cell 87, 757–766 (1996).
Dosanjh,M. K. et al. Isolation and characterization of RAD51C a new human member of the RAD51 family of related genes. Nucleic Acids Res. 26, 1179–1184 (1998).
Hays,S. L., Firmenich,A. A. & Berg,P. Complex formation in yeast double-strand break repair: Participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc. Natl Acad. Sci. USA 92, 6925–6929 (1995).
Johnson,R. D. & Symington,L. S. Functional differences and interactions among the putative RecA homologs Rad51, Rad55, and Rad57. Mol. Cell. Biol. 15, 4843–4850 (1995).
Sung,P. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 11, 1111–1121 (1997).
Vogelstein,B. & Kinzler,K. W. (eds) The Genetic Basis of Human Cancer (MacGraw-Hill, New York, 1998).
Kinzler,K. W. & Vogelstein,B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature 386, 761, 763 (1997).
Rouet,P., Smih,F. & Jasin,M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc. Natl Acad. Sci. USA 91, 6064–6068 (1994).
Donoho,G., Jasin,M. & Berg,P. Analysis of gene targeting and intrachromosomal homologous recombination stimulated by genomic double-strand breaks in mouse embryonic stem cells. Mol. Cell. Biol. 18, 4070–4078 (1998).
Acknowledgements
We thank L. Thompson and N. Jones for their assistance. This work was funded by an NRSA fellowship to R.D.J. a DOE grant (N.L.) and an NIH grant to M.J.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, R., Liu, N. & Jasin, M. Mammalian XRCC2 promotes the repair of DNA double-strand breaks by homologous recombination. Nature 401, 397–399 (1999). https://doi.org/10.1038/43932
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/43932
This article is cited by
-
Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets
Modern Pathology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.