Abstract
Sensing devices designed to detect explosive vapours are bulky, expensive and in need of technological improvement — dogs remain the most effective detectors1 in the fight against terrorism and in the removal of land-mines2,3. Here we demonstrate the deflagration of trinitrotoluene (TNT) in a small localized explosion on an uncoated piezoresistive microcantilever. This explosive-vapour sensor, which has a detection capability that is comparable to that of a dog, should enable extremely sensitive, miniature detection devices to be used on a large scale.
Similar content being viewed by others
Main
Microcantilevers with specific coatings can be used for chemical detection4,5, but TNT molecules, which are intrinsically 'sticky', readily adhere to uncoated cantilever surfaces6. When the source of TNT is removed, the TNT molecules slowly desorb. We found that applying a voltage pulse to an integrated piezoresistor during this desorption process causes the cantilever temperature to rise beyond the deflagration point of TNT, resulting in a miniature deflagration. Our cantilevers did not degrade after hundreds of pulses (10–25 volts) and deflagrations.
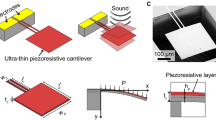
We monitored deflagration by capturing magnified high-speed video images, by measuring the deflection due to released heat using an optical beam-bounce technique that is used in atomic-force microscopy, and by measuring resonance-frequency shifts and heat added with a piezoelectric/piezoresistive cantilever7 operated in self-sensing mode by means of an a.c. bridge circuit8. The circuit's output increases with mass loading of adsorbed TNT and increasing temperature; it decreases with mass desorption and decreasing temperature.
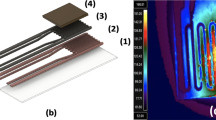
A five-event TNT-detection test, using the self-sensing platform described, is illustrated in Fig. 1. During event 1, before loading with TNT, a reference voltage pulse (25 volts) is applied to the piezoresistive heater, causing a temporary upward spike in circuit output that is due to heating. TNT loading (event 2) causes a gradual upward shift in sensor output, which then gradually decreases when the TNT begins to desorb from the cantilever (event 3). The second pulse (5 volts) during desorption does not raise the cantilever temperature sufficiently for deflagration (event 4). The third pulse (25 volts) causes deflagration, as shown by a visible smoke plume, and a dramatic mass decrease, which is verified by a reduction in circuit output (event 5) that overwhelms the upward thermal signal evident in event 1. Post-deflagration reference pulses of 25 volts resulted in spikes similar to the one seen in event 1.
a, Sensor output plotted against time. During event 1 (numbered arrow) of the test, a 25-volt reference pulse is sent to the piezoresistor before TNT loading begins; sensor output returns quickly to almost its pre-pulse value. In event 2, TNT is gradually loaded onto the cantilever and the resonance frequency decreases, causing the sensor output to increase. After loading, the TNT begins to desorb (event 3) and a 5-volt pulse (event 4) is insufficient to heat the explosive on the cantilever to its deflagration point. However, a 25-volt pulse is sufficient to cause deflagration (event 5). b, Left, diagram of the microcantilever, showing the integrated piezoelectric sensor, piezoresistive heater, and TNT loaded on the cantilever surface. Scale bar, 100 µm. Right, deflagration is confirmed in magnified high-speed video images; the smoke plume is visible, particularly when illuminated by red laser light.
The occurrence of deflagration was inferred from three consistent observations. First, the cantilever returns to its pre-test resonance frequency after deflagration, suggesting that all of the adsorbed material has been lost. Second, a specific voltage (corresponding to a threshold, or deflagration-point, temperature) is necessary to cause deflagration. Third, the measurement of heat added to the cantilever during deflagration shows that the reaction is exothermic, ruling out other possible reactions such as melting, vaporization or decomposition.
Our method currently detects the deflagration of as little as 70 picograms (1.9 × 1011 molecules) of TNT (calculated from the shift in cantilever resonance). This limit of detection is the same as that of an improved version9 of the ion-mobility mass-spectrometry technology now used for airport security. Calculations show that the detection limit could be improved by up to three orders of magnitude by using optimized cantilevers.
An explosive-detection technique based on deflagration is advantageous, because deflagration is a characteristic property of these substances. Other potentially interfering but non-explosive substances tested — including water, acetone, ethyl alcohol and gasoline — all desorbed in our system before the voltage pulse was applied. In the absence of a voltage pulse, desorption of nanogram quantities of deposited TNT takes tens of minutes; comparable amounts of water or alcohol desorb in seconds.
Other explosive molecules can be detected by this method and differentiated by their compound-specific deflagration points and desorption times (results not shown). Our technique could also be used in conjunction with coated-cantilever chemical-sensing arrays5, in which the arrays serve as an initial indicator, and positive readings are verified by deflagration on an uncoated cantilever.
References
Furton, K. G. & Myers, L. J. Talanta 54, 487–500 (2001).
Colton, R. J. & Russell, J. N. Science 299, 1324–1325 (2003).
Czarnik, A. W. Nature 394, 417–418 (1998).
Thundat, T., Chen, G. Y., Warmack, R. J., Allison, D. P. & Wachter, E. A. Anal. Chem. 67, 519–521 (1995).
Lang, H. et al. Appl. Phys. Lett. 72, 383 (1998).
Muralidharan, G. et al. Ultramicroscopy 97, 433–439 (2003).
Minne, S. C., Manalis, S. R. & Quate, C. F. Appl. Phys. Lett. 67, 3918–3920 (1995).
Rogers, B. et al. Rev. Sci. Instruments (in the press).
Khayamian, T., Tabrizchi, M. & Jafari, M.T. Talanta 59, 327–333 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
brief communications is intended to provide a forum for brief, topical reports of general scientific interest and for technical discussion of recently published material of particular interest to non-specialist readers (communications arising). Priority will be given to contributions that have fewer than 500 words, 10 references and only one figure. Detailed guidelines are available on Nature's website (http://www.nature.com/nature).
Rights and permissions
About this article
Cite this article
Pinnaduwage, L., Gehl, A., Hedden, D. et al. A microsensor for trinitrotoluene vapour. Nature 425, 474 (2003). https://doi.org/10.1038/425474a
Issue Date:
DOI: https://doi.org/10.1038/425474a
This article is cited by
-
Performance analysis of microcantilever array sensing
Science China Technological Sciences (2017)
-
Piezotransistive transduction of femtoscale displacement for photoacoustic spectroscopy
Nature Communications (2015)
-
Microcantilevers and organic transistors: two promising classes of label-free biosensing devices which can be integrated in electronic circuits
Analytical and Bioanalytical Chemistry (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.