Abstract
Flagellated bacteria swim up gradients of chemical attractants by modulating the direction of rotation of their flagellar motors, but how this switching mechanism works is not understood. Here we show that the probability of the motor rotating in the clockwise direction increases under high load, when the motor spins slowly (at less than 50 Hz). We suggest that either the switch is responding to small changes in torque — the torque increases only fractionally between 50 Hz and stall — or it senses motor speed, perhaps by means of proton flux.
Similar content being viewed by others
Main
Flagellar filaments of an Escherichia coli sticky-filament mutant1, which is otherwise wild-type for chemotaxis, were shortened by viscous shear, and cell bodies were fixed to a polylysine-coated glass coverslip. Latex beads of various sizes were adsorbed to filament stubs and their rotation was followed in a weak optical trap with a quadrant detector2.
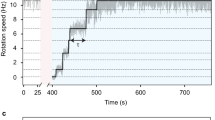
Figure 1 shows clockwise and counterclockwise intervals for rotation plotted as a function of counterclockwise speed, together with rate constants for transitions between the clockwise and counterclockwise states. Clockwise intervals lengthened appreciably at high loading (k− decreased), whereas counterclockwise intervals remained about the same for all loads (k+ remained roughly constant).
a, b, Mean intervals for clockwise (CW; a) and counterclockwise (CCW; b) rotation of the flagellar motor, plotted as a function of mean CCW speed. Filled symbols, values measured for each cell; hollow symbols, averages over the ensemble of cells for beads of a given size, with the values for each cell being weighted equally. Bead diameters were 0.36, 0.54, 0.74, 1.03, 1.20, 1.44, 1.79, 2.13 and 2.60 μm (from right to left); the number of cells studied for each of these bead sizes was 12, 12, 7, 12, 12, 18, 18, 16 and 10, respectively. Each bead was monitored for 5 min at 20 °C. Inset, rate constants k− = 1/(mean CW interval) and k+ = 1/(mean CCW interval) were computed for each cell and then averaged over the ensemble of cells for beads of a given size, with the values for each cell being weighted equally.
A similar asymmetrical performance is seen under the action of fumarate3. However, when an attractant is added, the cells respond by shortening their clockwise intervals and lengthening their counterclockwise intervals4. From the results shown in Fig. 1, it follows that a motor that is spinning rapidly, as motors do in swimming cells, will spend a larger fraction of its time turning counterclockwise and will change direction more frequently than a motor that is spinning slowly, as motors do in tethered cells.
In a wild-type cell, the direction in which the motor spins depends on the degree of phosphorylation of a small signalling protein, CheY, which is activated by a kinase that is coupled to the chemoreceptors5. To determine whether this signalling pathway is involved in the load response, we repeated a number of measurements using cells in which this pathway had been disrupted. We used a strain in which cheY, cheA (which encodes the kinase) and cheZ (which encodes a phosphatase) were all deleted and which expressed CheY13DK106YW (CheY**), a variant with fixed activity1. The response to different loadings was the same as that of wild-type cells, indicating that the motor's sensitivity to load is probably not due to feedback from the chemotaxis signalling network, but is instead an inherent property of the motor.
The bacterial flagellar motor is powered by protons moving down an electrochemical gradient and has a distinctive torque–speed relationship. At 20 °C, the relative torque falls gradually from unity at stall to about 0.9 at 120 Hz, and drops rapidly to zero at about 260 Hz (ref. 6); in the latter regime, the torque decreases markedly at lower temperatures or when H2O is replaced by D2O (ref. 7), indicating that the movement of mechanical parts and/or of protons becomes rate limiting.
We found switching to be particularly sensitive to load only during high-torque, low-speed operation, and mainly at speeds of less than about 50 Hz (Fig. 1). Over this speed range, the torque changes by about 5%. There is evidence that each revolution of the motor requires the passage of a fixed number of protons8. So, rather than sensing changes in torque, might the switch be monitoring proton flux?
Flagellated bacteria are sensitive to a variety of environmental factors, including mechanical stimuli. Swimming in viscous solutions or near surfaces can trigger developmental changes — for example, swarmer-cell differentiation in E. coli9 or lateral flagellar synthesis in the marine organism Vibrio10. In the latter case, changes in gene expression occur in response to exposure to sodium-channel blockers, indicating that they might be caused by a reduction in motor ion flux11. It is possible that these different responses rely on a common mechanosensory mechanism.
References
Scharf, B. E., Fahrner, K. A., Turner, L. & Berg, H. C. Proc. Natl Acad. Sci. USA 95, 201–206 (1998).
Ryu, W. S., Berry, R. M. & Berg, H. C. Nature 403, 444–447 (2000).
Prasad, K., Caplan, S. R. & Eisenbach, M. J. Mol. Biol. 280, 821–828 (1998).
Block, S. M., Segall, J. E. & Berg, H. C. J. Bacteriol. 154, 312–323 (1983).
Bourret, R. B. & Stock, A. M. J. Biol. Chem. 277, 9625–9628 (2002).
Chen, X. & Berg, H. C. Biophys. J. 78, 1036–1041 (2000).
Chen, X. & Berg, H. C. Biophys. J. 78, 2280–2284 (2000).
Meister, M., Lowe, G. & Berg, H. C. Cell 49, 643–650 (1987).
Harshey, R. M. Mol. Microbiol. 13, 389–394 (1994).
McCarter, L. L. Microbiol. Mol. Biol. Rev. 65, 445–462 (2001).
Kawagishi, I., Imagawa, M., Imae, Y., McCarter, L. & Homma, M. Mol. Microbiol. 20, 693–699 (1996).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fahrner, K., Ryu, W. & Berg, H. Bacterial flagellar switching under load. Nature 423, 938 (2003). https://doi.org/10.1038/423938a
Issue Date:
DOI: https://doi.org/10.1038/423938a
This article is cited by
-
Mechanosensitive recruitment of stator units promotes binding of the response regulator CheY-P to the flagellar motor
Nature Communications (2021)
-
Non-equilibrium effect in the allosteric regulation of the bacterial flagellar switch
Nature Physics (2017)
-
Impact of fluorescent protein fusions on the bacterial flagellar motor
Scientific Reports (2017)
-
Transient locking of the hook procures enhanced motility to flagellated bacteria
Scientific Reports (2017)
-
Running and tumbling with E. coli in polymeric solutions
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.