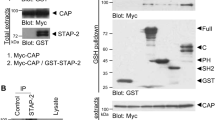
Abstract
The TRAP (thyroid hormone receptor-associated proteins) transcription coactivator complex (also known as Mediator) was first isolated as a group of proteins that facilitate the function of the thyroid hormone receptor1. This complex interacts physically with several nuclear receptors through the TRAP220 subunit, and with diverse activators through other subunits2. TRAP220 has been reported to show ligand-enhanced interaction with peroxisome proliferator-activated receptor γ2 (PPARγ2)3,4, a nuclear receptor essential for adipogenesis5,6,7,8. Here we show that Trap220-/- fibroblasts are refractory to PPARγ2-stimulated adipogenesis, but not to MyoD-stimulated myogenesis, and do not express adipogenesis markers or PPARγ2 target genes. These defects can be restored by expression of exogenous TRAP220. Further indicative of a direct role for TRAP220 in PPARγ2 function via the TRAP complex, TRAP functions directly as a transcriptional coactivator for PPARγ2 in a purified in vitro system and interacts with PPARγ2 in a ligand- and TRAP220-dependent manner. These data indicate that TRAP220 acts, via the TRAP complex, as a PPARγ2-selective coactivator and, accordingly, that it is specific for one fibroblast differentiation pathway (adipogenesis) relative to another (myogenesis).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fondell, J. D., Ge, H. & Roeder, R. G. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc. Natl Acad. Sci. USA 93, 8329–8333 (1996)
Malik, S. & Roeder, R. G. Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem. Sci. 25, 277–283 (2000)
Zhu, Y., Qi, C., Jain, S., Rao, M. S. & Reddy, J. K. Isolation and characterization of PBP, a protein that interacts with peroxisome proliferator-activated receptor. J. Biol. Chem. 272, 25500–25506 (1997)
Yuan, C. X., Ito, M., Fondell, J. D., Fu, Z. Y. & Roeder, R. G. The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc. Natl Acad. Sci. USA 95, 7939–7944 (1998)
Barak, Y. et al. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 (1999)
Kubota, N. et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 4, 597–609 (1999)
Rosen, E. D. et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617 (1999)
Lowell, B. B. PPARγ: an essential regulator of adipogenesis and modulator of fat cell function. Cell 99, 239–242 (1999)
Tontonoz, P., Hu, E. & Spiegelman, B. M. Stimulation of adipogenesis in fibroblasts by PPAR γ2, a lipid-activated transcription factor. Cell 79, 1147–1156 (1994)
Wu, Z. et al. Cross-regulation of C/EBPα and PPAR γ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 3, 151–158 (1999)
Glass, C. K. & Rosenfeld, M. G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 15, 121–141 (2000)
Gu, W. et al. A novel human SRB/MED-containing cofactor complex, SMCC, involved in transcription regulation. Mol. Cell 3, 97–108 (1999)
Ito, M. et al. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol. Cell 3, 361–370 (1999)
Jiang, Y. W. et al. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc. Natl Acad. Sci. USA 95, 8538–8543 (1998)
Malik, S., Gu, W., Wu, W., Qin, J. & Roeder, R. G. The USA-derived transcriptional coactivator PC2 is a submodule of TRAP/SMCC and acts synergistically with other PCs. Mol. Cell 5, 753–760 (2000)
Rachez, C. et al. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature 398, 824–828 (1999)
Sun, X. et al. NAT, a human complex containing Srb polypeptides that functions as a negative regulator of activated transcription. Mol. Cell 2, 213–222 (1998)
Naar, A. M. et al. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature 398, 828–832 (1999)
Boyer, T. G., Martin, M. E., Lees, E., Ricciardi, R. P. & Berk, A. J. Mammalian Srb/Mediator complex is targeted by adenovirus E1A protein. Nature 399, 276–279 (1999)
Ito, M., Yuan, C.-X., Okano, H. J., Darnell, R. B. & Roeder, R. G. Involvement of the TRAP220 component of the TRAP/SMCC coactivator complex in embryonic development and thyroid hormone action. Mol. Cell 5, 683–693 (2000)
Zhu, Y. et al. Deletion of PBP/PPARBP, the gene for nuclear receptor coactivator peroxisome proliferator-activated receptor-binding protein, results in embryonic lethality. J. Biol. Chem. 275, 14779–14782 (2000)
Todaro, G. J. & Green, H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17, 299–313 (1963)
Yeh, W. C., Cao, Z., Classon, M. & McKnight, S. L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 9, 168–181 (1995)
Novitch, B. G., Mulligan, G. J., Jacks, T. & Lassar, A. B. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J. Cell Biol. 135, 441–456 (1996)
Yang, W., Rachez, C. & Freedman, L. P. Discrete roles for peroxisome proliferator-activated receptor γ and retinoid X receptor in recruiting nuclear receptor coactivators. Mol. Cell. Biol. 20, 8008–8017 (2000)
Rosen, E. D. et al. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 16, 22–26 (2002)
Puigserver, P. et al. Activation of PPARγ coactivator-1 through transcription factor docking. Science 286, 1368–1371 (1999)
Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl Acad. Sci. USA 90, 8392–8396 (1993)
Ge, K. et al. Mechanism for elimination of a tumour suppressor: aberrant splicing of a brain-specific exon causes loss of function of Bin1 in melanoma. Proc. Natl Acad. Sci. USA 96, 9689–9694 (1999)
Guermah, M., Tao, Y. & Roeder, R. G. Positive and negative TAF(II) functions that suggest a dynamic TFIID structure and elicit synergy with traps in activator-induced transcription. Mol. Cell. Biol. 21, 6882–6894 (2001)
Acknowledgements
We thank S.O. Freytag for C/EBPβ cDNA and pWZLhygro plasmid; A.B. Lassar for pBabe-MyoD plasmid, anti-myogenin, anti-MyoD and anti-MHC antibodies; T.M. Wilson and S.A. Kliewer for rosiglitazone; G.P. Nolan for Phoenix retrovirus packaging cell lines; J. Zhang, S. Malik, U. Kim, X. Ren and other members of the laboratory for critical reading of the manuscript and discussion. This work was supported by the NIH. K.G. was supported by a postdoctoral fellowship from Charles H. Revson Foundation. A.E.W. was supported by the Swedish Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Ge, K., Guermah, M., Yuan, CX. et al. Transcription coactivator TRAP220 is required for PPARγ2-stimulated adipogenesis. Nature 417, 563–567 (2002). https://doi.org/10.1038/417563a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/417563a
This article is cited by
-
Regulation of the RNA polymerase II pre-initiation complex by its associated coactivators
Nature Reviews Genetics (2023)
-
Cdo1 promotes PPARγ-mediated adipose tissue lipolysis in male mice
Nature Metabolism (2022)
-
Med23 supports angiogenesis and maintains vascular integrity through negative regulation of angiopoietin2 expression
Communications Biology (2022)
-
Hyperleptinemia in obese state renders luminal breast cancers refractory to tamoxifen by coordinating a crosstalk between Med1, miR205 and ErbB
npj Breast Cancer (2021)
-
50+ years of eukaryotic transcription: an expanding universe of factors and mechanisms
Nature Structural & Molecular Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.