Abstract
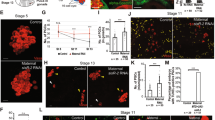
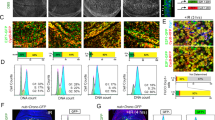
Embryogenesis is typically initiated by a series of rapid mitotic divisions that are under maternal genetic control1. The switch to zygotic control of embryogenesis at the midblastula transition is accompanied by significant increases in cell-cycle length and gene transcription, and changes in embryo morphology2,3. Here we show that mutations in the grapes (grp) checkpoint 1 kinase homologue4 in Drosophila block the morphological and biochemical changes that accompany the midblastula transition, lead to a continuation of the maternal cell-cycle programme, and disrupt DNA-replication checkpoint control of cell-cycle progression. The timing of the midblastula transition is controlled by the ratio of nuclei to cytoplasm (the nucleocytoplasmic ratio), suggesting that this developmental transition is triggered by titration of a maternal factor by the increasing mass of nuclear material that accumulates during the rapid embryonic mitoses5,6,7,8,9. Our observations support a model for cell-cycle control at the midblastula transition in which titration of a maternal component of the DNA-replication machinery slows DNA synthesis and induces a checkpoint-dependent delay in cell-cycle progression10. This delay may allow both completion of S phase and transcription of genes that initiate the switch to zygotic control of embryogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gilbert, S. F. Developmental Biology 3rd edn, 75–114 (Sinaur, Sunderland, MA, (1991)).
Newport, J. & Kirschner, M. Amajor developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 30, 675–686 (1982).
Foe, V. E., Odell, G. M. & Edgar, B. A. in The Development of Drosophila melanogaster(ed Bate, M. & Martinez Arias, A.) 149–300 (Cold Spring Harbor Laboratory Press, New York, (1993)).
Fogarty, P. et al. grp, a Drosphila gene with homology ot the S. pombe chk1/rad27 checkpoint gene, is required for the fidelity of the late syncytial divisions. Curr. Biol.(in the press).
Edgar, B. A., Kiehle, C. P. & Schubinger, G. Cell cycle control by the nucleo-cytoplasmic ratio in early Drosophila development. Cell 44, 365–372 (1986).
Yasuda, G. K., Baker, J. & Schubinger, G. Temporal regulation of gene expression in the blastoderm Drosophila embryo. Gen. Dev. 5, 1800–1812 (1991).
Pritchard, D. K. & Schubinger, G. Activation of transcription in Drosophila embryos is a gradual process mediated by the nucleocytoplasmic ratio. Gen. Dev. 10, 1131–1142 (1996).
Clute, P. & Masui, Y. Regulation of the appearance of division asynchrony and microtubule-dependent chromosome cycles in Xenopus laevis embryos. Dev. Biol. 171, 273–285 (1995).
Kane, D. A. & Kimmel, C. B. The zebrafish midblastula transition. Development 119, 447–456 (1993).
Dasso, M. & Newport, J. W. Completion of DNA replication is monitored by a feedback system that controls the initiaiton of mitosis in vitro: studies in Xenopus. Cell 61, 811–823 (1990).
Foe, V. E. & Alberts, B. M. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosphila embryogenesis. J. Cell Sci. 61, 31–71 (1983).
Sullivan, W., Fogarty, P. & Theurkauf, W. E. Mutations affecting the cytoskeletal organization of syncytial Drosophila embryos. Development 18, 1245–1254 (1993).
Matthies, H. J., McDonald, H. B., Goldstein, L. S. & Theurkauf, W. E. Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J. Cll Biol. 341, 455–464 (1996).
Kellogg, D. R., Mitchison, T. J. & Alberts, B. M. Behavior of microtubules and actin filaments in living Drosophila embryos. Development 103, 675–686 (1988).
Edgar, B. A., Sprenger, F., Duronio, R. J., Leopold, P. & O'Farrell, P. H. Distinct molecular mechanisms regulate cell cycle timing at successive stages of Drosophila embryogenesis. Gen. Dev. 8, 440–452 (1994).
Elledge, S. J. Cell cycle checkpoint: preventing an identity crisis. Science 274, 1664–1672 (1996).
Edgar, B. A. & Datar, S. A. Zygotic degradation of two maternal Cdc25 mRNAs terminates Drosophila's early cell cycle program. Gen. Dev. 10, 1966–1977 (1996).
Campbell, S. D., Sprenger, F., Edgar, B. A. & O'Farrel, P. H. Drosophila Wee 1 kinase rescues fission yeast from mitotic catastrophe and phosphorylates Drosophila Ccd2 in nitro. Mol. Biol. Cell 6, 1333–1347 (1995).
Klinger, M. & Gergen, J. P. Regulation of runt transcription by Drosophila segmentation genes. Mech. Dev. 43, 3–19 (1993).
Cooley, L., Kelley, R. & Spradling, A. Insertional mutagenesis of the Drosophila genome with single P elements. Science 239, 1121–1128 (1988).
Fogarty, P., Kalpin, R. F. & Sullivan, W. The Drosophila maternal-effect mutation grapes causes a metaphase arrest at nuclear cycle 13. Development 120, 2131–2142 (1994).
Francesconi, S., Grenon, M., Bouvier, D. & Baldacci, G. p56chk1protein kinase is required for the DNA replication checkpoint at 37 °C in fission yeast. EMBO J. 16, 1332–1341 (1997).
Walworth, N. C. & Bernards, R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science 271, 353–356 (1996).
Raff, J. W. & Glover, D. M. Nuclear and cytoplasmic cycles continue in Drosophila embryos in which DNA synthesis is inhibited with aphidicolin. J. Cell Biol. 107, 2009–2019 (1988).
Banga, S. S., Shenkar, R. & Boyd, J. B. Hypersensitivity of Drosophila mei-41 mutants to hydroxyurea is associated with reduced mitotic chromosome stabiity. Mutant. Res. 163, 157–165 (1986).
Shermoen, A. W. & O'Farrell, P. H. Progression of the cell cycle through mitosis leads to abortion of nascent transcripts. Cell 67, 303–310 (1991).
Ruden, D. M. & Jackle, H. Mitotic delay dependent survival identifies components of cell cycle control in the Drosophila blastoderm. Development 121, 63–73 (1995).
Theurkauf, W. E. in Methods of Cell Biology: Drosophila melanogaster: Practical Uses in Cell and Molecular Biology(eds Goldstein, L. S. B. & Fryberg, E.) 489–505 (Academic, New York, (1994).
Tautz, D., Pfeifle, C. Anon-radioactive in situ hybridization method for the localization of specific RNA's in Drosophila embryo's reveals translational control of the segmentation gene hunchback. Chromosoma 98, 81–85 (1989).
Acknowledgements
We thank W. Sullivan, S. Campbell, B. Holdener, J. Kramer, N. Hollingsworth and B. Eggen for comments on the manuscript; B. Edgar and P. O'Farrell for discussions; P. Gergen for probes and advice on whole-mount in situ hybridization; P. Fogarty and W. Sullivan for sharing unpublished data and providing grp cDNA clones; R. S. Hawley and colleagues at the University of California at Davis for providing their collection of P-element-associated maternal-effect lethal mutations; and B. Edgar for anti-Cdc2 antibodies and advice on cell-cycle control during early embryogenesis. This work was supported by a fellowship from the NWO to O.C.M.S. and grants from the NIH and American Cancer Society to W.E.T.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sibon, O., Stevenson, V. & Theurkauf, W. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature 388, 93–97 (1997). https://doi.org/10.1038/40439
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/40439
This article is cited by
-
Selective translation of epigenetic modifiers affects the temporal pattern and differentiation of neural stem cells
Nature Communications (2022)
-
Mutations in the insulator protein Suppressor of Hairy wing induce genome instability
Chromosoma (2020)
-
The neglected part of early embryonic development: maternal protein degradation
Cellular and Molecular Life Sciences (2020)
-
Impact of gut microbiota on the fly’s germ line
Nature Communications (2016)
-
REGULATOR: a database of metazoan transcription factors and maternal factors for developmental studies
BMC Bioinformatics (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.