Abstract
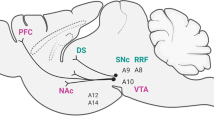
In this review, we will examine the most recent preclinical evidence in support of the fact that both acute and chronic stress may have a detrimental impact on the normal function of the dopaminergic system. In recent decades, the term stress has changed its meaning from that of a ‘non-specific body response’ to a ‘monitoring system of internal and external cues’; that is a modality of reaction of the mammalian central nervous system (CNS) which is critical to the adaptation of the organism to its environment. Compelling results have demonstrated that the dopaminergic system is important not only for hedonic impact or reward learning but also, in a broader sense, for reactivity to perturbation in environmental conditions, for selective information processing, and for general emotional responses, which are essential functions in the ability (or failure) to cope with the external world. In this, stress directly influences several basic behaviors which are mediated by the dopaminergic system such as locomotor activity, sexual activity, appetite, and cross sensitization with drugs of abuse. Studies using rat lines which are genetically different in dopamine (DA) physiology, have shown that even small alterations in the birth procedure or early life stress events may contribute to the pathophysiology of psychiatric disorders—in particular those involving central DA dysfunction—and may cause depression or psychotic derangement in the offspring. Finally, the fact that the dopaminergic system after stress responds, preferentially, in the medial prefrontal cortex (MFC), is thought to serve, in humans, as a protection against positive psychotic symptoms, since the increased DA activity in the MFC suppresses limbic DA transmission. However, excessive MFC dopaminergic activity has a negative impact on the cognitive functions of primates, making them unable to select and process significant environmental stimuli. Thus it appears that a critical range of DA turnover is necessary for optimal cognitive functioning after stress, in the response of the CNS to ever-changing environmental demands.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Selye H . The evolution of the stress concept Am Sci 1973; 61: 692–696
Akil HA, Morano IM . Stress. In: Kupfer D, Bloom F (eds) Psychopharmacology, the Fourth Generation of Progress Raven Press: New York 1995; pp773–785
Pani L, Gessa GL . Evolution of the dopaminergic system and its relationships with the psychopathology of pleasure Int J Clin Pharm Res 1997; 17: 55–58
Berridge KC, Robinson TE . What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 1998; 28: 309–369
Wise RA . The brain and reward. In: Liebman J, Cooper SJ (eds) The Neuropharmacological Basis of Reward Oxford University Press: Oxford 1989; pp377–424
Fibiger HC, Phillips AG . Role of catecholamine transmitters in reward systems: implications for the neurobiology of affect. In: Oreland E (ed) Brain Reward Systems and Abuse New York Press: New York 1987; pp61–74
Blackburn JR, Pfaus JG, Phillips AG . Dopamine functions in appetitive and defensive behaviors Progr Neurobiol 1992; 39: 247–279
Kaneyuki H, Yokoo H, Tsuda A, Yoshida M, Mizuki Y, Yamada M et al. Psychological stress increases dopamine turnover selectively in mesoprefrontal dopamine neurons of rats: reversal by diazepam Brain Res 1991; 557: 154–161
Doherty MD, Gratton A . NMDA receptors in nucleus accumbens modulate stress-induced dopamine release in nucleus accumbens and ventral tegmental area Synapse 1997; 26: 225–234
King D, Zigmond MJ, Finlay JM . Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell Neuroscience 1997; 77: 141–153
King D, Finlay JM . Loss of dopamine terminals in the medial prefrontal cortex increased the ratio of DOPAC to DA in tissue of the nucleus accumbens shell: role of stress Brain Res 1997; 767: 192–200
Griffiths J, Shanks N, Anisman H . Strain-specific alterations in consumption of a palatable diet following repeated stressor exposure Pharmacol Biochem Behav 1992; 42: 219–227
Sato Y, Kumamoto Y . Psychological stress and sexual behavior in male rats. II. Effect of psychological stress on dopamine and its metabolites in the critical brain areas mediating sexual behavior Nippon Hinyokika Gakkai Zasshi 1992; 83: 212–219
Wang CT, Huang RL, Tai MY, Tsai YF, Peng MT . Dopamine release in the nucleus accumbens during sexual behavior in prenatally stressed adult male rats Neurosci Lett 1995; 200: 29–32
Sugiura K, Yoshimura H, Yokoyama M . An animal model of copulatory disorder induced by social stress in male mice: effects of apomorphine and L. dopa Psychopharmacol Berl 1997; 133: 249–255
Brake WG, Noel MB, Boksa P, Gratton A . Influence of perinatal factors on the nucleus accumbens dopamine response to repeated stress during adulthood: an electrochemical study in the rat Neuroscience 1997; 77: 1067–1076
Alonso SJ, Navarro E, Rodriguez M . Permanent dopaminergic alterations in the n. accumbens after prenatal stress Pharmacol Biochem Behav 1994; 49: 353–358
Zebrowska-Lupina I, Stelmasiak M, Porowska A . Stress, induced depression of basal motility: effects of antidepressant drugs Pol J Pharmacol Pharm 1990; 42: 97–104
Sampson D, Willner P, Muscat R . Reversal of antidepressant action by dopamine antagonists in an animal model of depression Psychopharmacol Berl 1991; 104: 491–495
Zebrowscka-Lupina I, Ossowska G, Klenk-Majewska B . The influence of antidepressants on aggressive behavior in stressed rats: the role of dopamine Pol J Pharmacol Pharm 1992; 44: 325–335
Sampson D, Willner P, Muscat R . Reversal of antidepressant action by dopamine antagonists in an animal model of depression Psychopharmacol Berl 1991; 104: 491–495
Muscat R, Papp M, Willner P . Reversal of stress, induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline Psychopharmacol Berl 1992; 109: 433–438
Papp M, Willner P, Muscat R . Behavioural sensitization to a dopamine agonist is associated with reversal of stress, induced anhedonia Psychopharmacol Berl 1993; 110: 159–164
Papp M, Muscat R, Willner P . Subsensitivity to rewarding and locomotor stimulant effects of a dopamine agonist following chronic mild stress Psychopharmacol Berl 1993; 110: 152–158
Willner P . Pharmacology of anhedonia Eur Neuropsychopharmacol 1995; 5: (suppl 3) 214s–221s
Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH . Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys Proc Natl Acad Sci USA 1996; 93: 1325–1329
Murphy BL, Arnsten AF, Jentsch JD, Roth RH . Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress, induced impairment J Neurosci 1996; 16: 7768–7775
Steketee JD, Kalivas PW . Sensitization to psychostimulants and stress after injection of pertussis toxin into the A10 dopamine region J Pharmacol Exp Ther 1991; 259: 916–924
Sorg BA, Steketee JD . Mechanisms of cocaine, induced sensitization Prog Neuropsychopharmacol Biol Psychiatry 1992; 16: 1003–1012
Meiergerd SM, Schenk JO, Sorg BA . Repeated cocaine and stress increase dopamine clearance in the rat medial prefrontal cortex Brain Res 1997; 773: 203–207
Zahrt J, Taylor JR, Mathew RG, Arnsten AF . Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance J Neurosci 1997; 17: 8528–8535
Deutch AY, Clark WA, Roth RH . Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress Brain Res 1990; 521: 311–315
Friedhoff AJ, Carr KD, Uysal S, Schweitzer J . Repeated inescapable stress produces a neuroleptic-like effect on the conditioned avoidance response Neuropsychopharmacology 1995; 13: 129–138
Finlay JM, Zigmond MJ . The effect of stress on central dopaminergic neurons: possible clinical implications Neurochem Res 1997; 22: 1387–1394
MacLean PD . Brain evolution relating to family, play and the separation call Arch Gen Psychiatry 1985; 42: 405–417
Cenci MA, Kalen P, Mandel RJ, Bjorklund A . Regional differences in the regulation of dopamine and noradrenaline release in medial frontal cortex, nucleus accumbens and caudate-putamen: a microdialysis study in the rat Brain Res 1992; 581: 217–228
Cabib S, Puglisi-Allegra S . Stress, depression and the mesolimbic dopamine system Psychopharmacol Berl 1996; 128: 331–342
Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ . Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex Cereb Cortex 1991; 1: 273–292
Keefe KA, Stricker EM, Zigmond MJ, Abercrombie ED . Environmental stress increases extracellular dopamine in striatum of 6-hydroxydopamine-treated rats: in vivo microdialysis studies Brain Res 1990; 527: 350–353
Tidey JW, Miczek KA . Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study Brain Res 1996; 721: 140–149
Carlson JN, Fitzgerald LW, Keller RW Jr, Glick SD . Side and region dependent changes in dopamine activation with various durations of restraint stress Brain Res 1991; 550: 313–318
Cabib S, Puglisi-Allegra S . Genotype-dependent effects of chronic stress on apomorphine-induced alterations of striatal and mesolimbic dopamine metabolism Brain Res 1991; 542: 91
Inoue T, Tsuchiya K, Koyama T . Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain Pharmacol Biochem Behav 1994; 49: 911–920
Deutch AY, Cameron DS . Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell Neuroscience 1992; 46: 49–56
Deutch AY . Prefrontal cortical dopamine systems and the elaboration of functional corticostriatal circuits: implications for schizophrenia and Parkinson's disease J Neural Transm Gen Sect 1993; 91: 197–221
Kalivas PW, Duffy P . Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress Brain Res 1995; 675: 325–328
Jordan S, Kramer GL, Zukas PK, Petty F . Previous stress increases in vivo biogenic amine response to swim stress Neurochem Res 1994; 19: 1521–1525
Gresch PJ, Sved AF, Zigmond MJ, Finlay JM . Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat J Neurochem 1994; 63: 575–583
Sudha S, Pradhan N . Stress, induced changes in regional monoamine metabolism and behavior in rats Physiol Behav 1995; 57: 1061–1066
Shanks N, Zalcman S, Zacharko RM, Anisman H . Alterations of central norepinephrine, dopamine and serotonin in several strains of mice following acute stressor exposure Pharmacol Biochem Behav 1991; 38: 69–75
Puglisi-Allegra S, Kempf E, Cabib S . Role of genotype in the adaptation of the brain dopamine system to stress Neurosci Biobehav Rev 1990; 14: 523–528
Cabib S, Puglisi-Allegra S . Genotype-dependent effects of chronic stress on apomorphine-induced alterations of striatal and mesolimbic dopamine metabolism Brain Res 1991; 542: 91–96
Puglisi-Allegra S, Kempf E, Schleef C, Cabib S . Repeated stressful experiences differently affect brain dopamine receptor subtypes Life Sci 1991; 48: 1263–1268
Badiani A, Cabib S, Puglisi-Allegra S . Chronic stress induces strain-dependent sensitization to the behavioral effects of amphetamine in the mouse Pharmacol Biochem Behav 1992; 43: 53–60
Kamei H, Kameyama T, Nabeshima T . Activation of both dopamine D1 and D2 receptors necessary for amelioration of conditioned fear stress Eur J Pharmacol 1995; 273: 229–333
Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le-Moal M et al. Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens Brain Res 1995; 685: 179–186
Kiyatkin EA, Belyi VP, Rusakov DYU, Maksimov VV, Pankratova NV, Rozhanets VV . Long-term changes of striatal D-2 receptors in rats chronically exposed to morphine under aversive life conditions Int J Neurosci 1991; 58: 55–61
Tomic M, Joksimovic J . Glucocorticoid status affects the response of rat striatal dopamine D2 receptors to hyperthermia and turpentine treatment Endocr Regul 1991; 25: 225–230
Papp M, Klimek V, Willner P . Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress, induced anhedonia and its reversal by imipramine Psychopharmacol Berl 1994; 115: 441–446
Steketee JD, Kalivas PW . Sensitization to psychostimulants and stress after injection of pertussis toxin into the A10 dopamine region J Pharmacol Exp Ther 1991; 259: 916–924
Fontenot MB, Kaplan JR, Manuck SB, Arango V, Mann JJ . Long-term effects of chronic social stress on serotonergic indices in the prefrontal cortex of adult male cynomolgus macaques Brain Res 1995; 705: 105–108
Kelland MD, Chiodo LA . Serotonergic modulation of midbrain dopamine systems. In: Ashby CA Jr (ed) The Modulation of Dopaminergic Neurotransmission by Other Neurotransmitters CRC Press: Boca Raton 1996; pp87–122
Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA . Social stress, depression, and brain dopamine in female cynomolgus monkeys Ann NY Acad Sci 1997; 15: 574–577
Feenstra MG, Kalsbeek A, Van-Galen H . Neonatal lesions of the ventral tegmental area affect monoaminergic responses to stress in the medial prefrontal cortex and other dopamine projection areas in adulthood Brain Res 1992; 596: 169–182
Cabib S, Puglisi-Allegra S, D'Amato FR . Effects of postnatal stress on dopamine mesolimbic system responses to aversive experiences in adult life Brain Res 1993; 604: 232–239
Lipska BK, Chrapusta SJ, Egan MF, Weinberger DR . Neonatal excitotoxic ventral hippocampal damage alters dopamine response to mild repeated stress and to chronic haloperidol Synapse 1995; 20: 125–130
Inglefield JR, Kellogg CK . Hypothalamic GABAA receptor blockade modulates cerebral cortical systems sensitive to acute stressors Psychopharmacol Berl 1994; 116: 339–345
Biggio G, Concas A, Corda MG, Giorgi O, Sanna E, Serra M . GABAergic and dopaminergic transmission in the rat cerebral cortex: effect of stress, anxiolytic and anxiogenic drugs Pharmacol Ther 1990; 48: 121–142
Boireau A, Dubedat P, Laduron PM, Doble A, Blanchard JC . Preferential decrease in dopamine utilization in prefrontal cortex by zopiclone, diazepam and zolpidem in unstressed rats J Pharm Pharmacol 1990; 42: 562–565
Kaneyuki H, Yokoo H, Tsuda A, Yoshida M, Mizuki Y, Yamada M et al. Psychological stress increases dopamine turnover selectively in mesoprefrontal dopamine neurons of rats: reversal by diazepam Brain Res 1991; 557: 154–161
Grobin AC, Roth RH, Deutch AY . Regulation of the prefrontal cortical dopamine system by the neuroactive steroid 3a,21-dihydroxy-5a-pregnane-20-one Brain Res 1992; 578: 351–356
Hegarty AA, Vogel WH . Modulation of the stress response by ethanol in the rat frontal cortex Pharmacol Biochem Behav 1993; 45: 327–334
Dazzi L, Motzo C, Imperato A, Serra M, Gessa GL, Biggio G . Modulation of basal and stress-induced release of acetylcholine and dopamine in rat brain by abecarnil and imidazenil, two anxioselective gamma-aminobutyric acidA receptor modulators J Pharmacol Exp Ther 1995; 273: 241–247
Wedzony K, Mackowiak M, Fijal K, Golembiowska K . Evidence that conditioned stress enhances outflow of dopamine in rat prefrontal cortex: a search for the influence of diazepam and 5-HT1A agonists Synapse 1996; 24: 240–247
Finlay JM, Zigmond MJ . The effect of stress on central dopaminergic neurons: possible clinical implications Neurochem Res 1997; 22: 1387–1394
Morrow BA, Clark WA, Roth RH . Stress activation of mesocorticolimbic dopamine neurons: effects of a glycine/NMDA receptor antagonist Eur J Pharmacol 1993; 238: 255–262
Goldstein LE, Rasmusson AM, Bunney BS, Roth RH . The NMDA glycine site antagonist (+)-HA-966 selectively regulates conditioned stress-induced metabolic activation of the mesoprefrontal cortical dopamine but not serotonin systems: a behavioral, neuroendocrine, and neurochemical study in the rat J Neurosci 1994; 14: 4937–4950
Keefe KA, Sved AF, Zigmond MJ, Abercrombie ED . Stress-induced dopamine release in the neostriatum: evaluation of the role of action potentials in nigrostriatal dopamine neurons or local initiation by endogenous excitatory amino acids J Neurochem 1993; 61: 1943–1952
Jedema HP, Moghaddam B . Glutamatergic control of dopamine release during stress in the rat prefrontal cortex J Neurochem 1994; 63: 785–788
Doherty MD, Gratton A . NMDA receptors in nucleus accumbens modulate stress-induced dopamine release in nucleus accumbens and ventral tegmental area Synapse 1997; 26: 225–234
Jaskiw GE, Karoum FK, Weinberger DR . Persistent elevations in dopamine and its metabolites in the nucleus accumbens after mild subchronic stress in rats with ibotenic acid lesions of the medial prefrontal cortex Brain Res 1990; 534: 321–323
Hutson PH, Barton CL . L-701,324, a glycine/NMDA receptor antagonist, blocks the increase of cortical dopamine metabolism by stress and DMCM Eur J Pharmacol 1997; 326: 127–132
Piazza PV, Le Moal MI . The role of stress in drug self-administration TIPS 1998; 19: 67–74
De-Kloet ER, Rots NY, Cools AR . Brain-corticosteroid hormone dialogue: slow and persistent Cell Mol Neurobiol 1996; 16: 345–356
Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le Moal M et al. Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens Brain Res 1995; 685: 179–186
Alonso SJ, Navarro E, Rodriguez M . Permanent dopaminergic alterations in the n. accumbens after prenatal stress Pharmacol Biochem Behav 1994; 49: 353–358
Piazza PV, Le Moal ML . Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons Annu Rev Pharmacol Toxicol 1996; 36: 359–378
Steketee JD, Kalivas PW . Sensitization to psychostimulants and stress after injection of pertussis toxin into the A10 dopamine region J Pharmacol Exp Ther 1991; 259: 916–924
Rouge Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV . Stress, induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress, induced corticosterone secretion J Neurosci 1995; 15: 7189–7995
Prasad BM, Sorg BA, Ulibarri C, Kalivas PW . Sensitization to stress and psychostimulants. Involvement of dopamine transmission versus the HPA axis Ann NY Acad Sci 1995; 771: 617–625
Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV . Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion J Neurosci 1995; 15: 7181–7188
Sorg BA, Kalivas PW . Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum Brain Res 1991; 559: 29–36
Sorg BA, Kalivas PW . Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex Neuroscience 1993; 53: 695–703
Hamamura T, Fibiger HC . Enhanced stress-induced dopamine release in the prefrontal cortex of amphetamine-sensitized rats Eur J Pharmacol 1993; 237: 65–71
Diaz Otanez CS, Capriles NR, Cancela LM . D1 and D2 dopamine and opiate receptors are involved in the restraint stress-induced sensitization to the psychostimulant effects of amphetamine Pharmacol Biochem Behav 1997; 58: 9–14
Hegarty AA, Vogel WH . Modulation of the stress response by ethanol in the rat frontal cortex Pharmacol Biochem Behav 1993; 45: 327–334
Matsuguchi N, Ida Y, Shirao I, Tsujimaru S . Blocking effects of ethanol on stress, induced activation of rat mesoprefrontal dopamine neurons Pharmacol Biochem Behav 1994; 48: 297–299
Koechling UM, Amit Z . Effects of CA antagonists on ethanol-induced excitation in habituated and nonhabituated mice: interaction with stress factors? Pharmacol Biochem Behav 1993; 44: 791–796
Berman SM, Noble EP . The D2 dopamine receptor (DRD2) gene and family stress; interactive effects on cognitive functions in children Behav Genet 1997; 27: 33–43
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pani, L., Porcella, A. & Gessa, G. The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry 5, 14–21 (2000). https://doi.org/10.1038/sj.mp.4000589
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4000589
Keywords
This article is cited by
-
The cancer-immune dialogue in the context of stress
Nature Reviews Immunology (2024)
-
Anhedonia in Depression: Neurobiological and Genetic Aspects
Neuroscience and Behavioral Physiology (2022)
-
Stress and the dopaminergic reward system
Experimental & Molecular Medicine (2020)
-
Migration and schizophrenia: meta-analysis and explanatory framework
European Archives of Psychiatry and Clinical Neuroscience (2020)
-
Methyl jasmonate reverses chronic stress-induced memory dysfunctions through modulation of monoaminergic neurotransmission, antioxidant defense system, and Nrf2 expressions
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)