Abstract
Kaposi's sarcoma–associated herpesvirus, the viral agent of Kaposi's sarcoma, is associated with two lymphoproliferative disorders: primary effusion lymphoma and multicentric Castleman's disease. To identify other lymphoproliferative conditions linked with Kaposi's sarcoma–associated herpesvirus, we studied non-Hodgkin's lymphomas arising in individuals with AIDS-associated Kaposi's sarcoma. Formalin-fixed tissues from 24 such lymphomas were examined. As expected, two primary effusion lymphomas were Kaposi's sarcoma–associated herpesvirus–positive, with immunohistochemistry demonstrating the Kaposi's sarcoma–associated herpesvirus latency-associated nuclear antigen in the nuclei of all neoplastic cells. Additionally, three of seven evaluable cases of the immunoblastic variant of diffuse large B-cell lymphoma (immunoblastic lymphoma) showed similar latency-associated nuclear antigen staining. These Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas resembled primary effusion lymphoma histologically but were not known to involve body cavities (sites included lymph nodes, soft tissues of the neck, and spleen). Notably, 5–20% of the neoplastic cells in the Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas also showed cytoplasmic staining for viral interleukin-6, a biologically active cytokine homologue found in primary effusion lymphoma. We conclude that Kaposi's sarcoma–associated herpesvirus is present in some immunoblastic lymphomas in persons with AIDS-associated Kaposi's sarcoma.
Similar content being viewed by others
INTRODUCTION
The viral agent of Kaposi's sarcoma, Kaposi's sarcoma–associated herpesvirus (also known as human herpesvirus 8), may cause non-Hodgkin's lymphoma. Kaposi's sarcoma–associated herpesvirus is universally present in primary effusion lymphoma cells (1, 2). Histologically, primary effusion lymphoma resembles the immunoblastic variant of diffuse large B-cell lymphoma (i.e., immunoblastic lymphoma) but uniquely involves serous body cavities (1). Additionally, Kaposi's sarcoma–associated herpesvirus is found in a subset of multicentric Castleman's disease cases and in plasmablastic lymphomas that can arise during multicentric Castleman's disease (3, 4). Primary effusion lymphoma and multicentric Castleman's disease occur most commonly in human immunodeficiency virus infection/acquired immunodeficiency syndrome (HIV/AIDS), although even in that setting they are rare (5, 6).
Kaposi's sarcoma–associated herpesvirus is less clearly involved in other non-Hodgkin's lymphoma types. In large pathology-based studies (2, 7, 8, 9), 0–22% of AIDS-associated immunoblastic lymphomas were Kaposi's sarcoma–associated herpesvirus positive by polymerase chain reaction (2, 7, 8, 9). However, these studies had very few AIDS-associated immunoblastic lymphomas, and Kaposi's sarcoma–associated herpesvirus appeared to be present at a low level in positive specimens, leaving its role uncertain. More recently, Kaposi's sarcoma–associated herpesvirus was detected by immunohistochemistry and polymerase chain reaction in additional cases of extracavitary lymphoma, described histologically as diffuse large B-cell lymphoma or immunoblastic lymphoma, in persons with multicentric Castleman's disease or HIV/AIDS (4, 10).
We previously used registry data to study cancers occurring in persons with AIDS-associated Kaposi's sarcoma, using the presence of Kaposi's sarcoma as a marker for Kaposi's sarcoma–associated herpesvirus infection (11). In that study, individuals with Kaposi's sarcoma had significantly increased risk for immunoblastic lymphoma (relative risk, 2.44; 95% CI, 2.00–2.96, compared with others with AIDS). The association persisted even after adjustment for possible confounding factors (adjusted relative risk, 1.58; 95% CI, 1.29–1.93) and was not present for other subtypes of non-Hodgkin's lymphoma. Our analyses thus suggested that Kaposi's sarcoma–associated herpesvirus might be etiologically important in a subset of AIDS-associated immunoblastic lymphomas. Notably, only 1 of the 151 immunoblastic lymphomas in persons with Kaposi's sarcoma arose in the pleura or peritoneum (11), implying that the link between immunoblastic lymphoma and Kaposi's sarcoma was not explained wholly by the occurrence of primary effusion lymphoma.
The phenotypic spectrum and incidence of Kaposi's sarcoma–associated herpesvirus–positive non-Hodgkin's lymphomas are not well characterized. In the present investigation, we systematically examined non-Hodgkin's lymphomas from HIV-infected individuals who also had a diagnosis of Kaposi's sarcoma. We hypothesized that because persons with AIDS-associated Kaposi's sarcoma control Kaposi's sarcoma–associated herpesvirus infection poorly, they might be especially prone to Kaposi's sarcoma–associated herpesvirus-induced non-Hodgkin's lymphoma.
METHODS
From the National Cancer Institute's AIDS Cancer Specimen Bank (1982–2001) and the pathology department of University of California, Los Angeles (1994–2001), we retrieved formalin-fixed, paraffin-embedded tumor tissue from 24 non-Hodgkin's lymphomas arising in HIV-infected persons who also had Kaposi's sarcoma at some point in their clinical course. For 14 cases, tissues were obtained at autopsy, resulting in decreased viability of tumor cells.
Immunohistochemical stains of tissue sections were performed using antibodies against CD20 (DAKO, Carpinteria, CA), CD3 (Dako), CD38 (Novocastra, Vector Laboratories, Burlingame, CA), and κ and λ immunoglobulin (Ig) light chains (DAKO). κ and λ Ig stains were scored as negative when tumor cells appeared negative in the presence of adequate staining of background plasma cells. Unsatisfactory was defined as absence of staining caused by poor antigenic preservation or, in the case of κ and λ, nonspecific staining of the cytoplasm of tumor cells for both light chains caused by nonspecific uptake of serum proteins. This latter pattern was most commonly seen in tumor specimens obtained at autopsy.
Non-Hodgkin's lymphomas were categorized using the World Health Organization classification (12) by two hematopathologists (SP and ESJ). Because the distinction between immunoblastic and plasmablastic variants of diffuse large B-cell lymphoma is sometimes ambiguous, subclassification was based on a combination of cytological and immunophenotypic criteria. Plasmablastic lymphomas had abundant basophilic cytoplasm, eccentric nuclei, and prominent central nucleoli. The tumor cells tended to have a cohesive growth pattern and were relatively uniform in cellular appearance. Plasmablastic lymphomas were by definition CD20 negative and CD38 and cytoplasmic Ig positive. Immunoblastic lymphomas were more pleomorphic and variable in appearance but exhibited basophilic cytoplasm and large nuclei with prominent nucleoli. Immunoblastic lymphomas usually expressed CD20 and had variable expression of CD38 and Ig.
We identified Kaposi's sarcoma–associated herpesvirus-infected cells using a rat monoclonal antibody against the Kaposi's sarcoma–associated herpesvirus latency-associated nuclear antigen (Advanced Biotechnologies, Columbia, MD). Additionally, we stained for viral interleukin-6, a Kaposi's sarcoma–associated herpesvirus cytokine homologue, using mouse monoclonal antibody v6m12.1.1 (13). Epstein Barr virus was detected by in situ hybridization with an EBER-1 RNA riboprobe.
RESULTS
Of the 24 non-Hodgkin's lymphoma cases, all but three were extranodal, with sites including brain (6 cases), lung (3 cases), liver (3 cases), pericardium (2 cases), or other (7 cases; see Table 1). As shown in Table 1, 20 cases (83%) were diffuse large B-cell lymphomas, further subclassified as immunoblastic (10 cases), centroblastic (8 cases), or plasmablastic (2 cases) variants. Other non-Hodgkin's lymphomas were classified as primary effusion lymphoma (2 cases), Burkitt's lymphoma (1 case), and aggressive B-cell non-Hodgkin's lymphoma, not otherwise subtyped (1 case). Foci of Kaposi's sarcoma were present in sections adjacent to two immunoblastic lymphomas (Cases 8 and 9), and an additional immunoblastic lymphoma was associated with both multicentric Castleman's disease and Kaposi's sarcoma (Case 1, see below). Kaposi's sarcoma was not seen in sections from other lymphomas.
Immunoblastic lymphomas were composed of a proliferation of transformed lymphoid cells with prominent central nucleoli and relatively abundant amphophilic cytoplasm. Three immunoblastic lymphomas (Cases 1, 2, and 9) showed more pronounced plasmacytoid differentiation, with frequent binucleation and occasional multinucleation. Immunoblastic lymphomas generally showed greater pleomorphism and a broader range in cell size, when compared with the plasmablastic variant of diffuse large B-cell lymphoma. Both immunoblastic and plasmablastic diffuse large B-cell lymphomas were often CD38 positive, and loss of CD20 was associated with plasmablastic features and plasmacytoid differentiation. Centroblastic diffuse large B-cell lymphomas involved extranodal sites, were often associated with necrosis, and tended to be CD20 positive and CD38 negative.
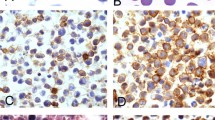
Three of seven evaluable immunoblastic lymphomas were positive for Kaposi's sarcoma–associated herpesvirus, with the tumors demonstrating nuclear staining for latency-associated nuclear antigen in all neoplastic cells (Fig. 1). Similar Kaposi's sarcoma–associated herpesvirus staining was seen in both primary effusion lymphomas. All other non-Hodgkin's lymphomas were negative for Kaposi's sarcoma–associated herpesvirus. Thirteen of 21 evaluable non-Hodgkin's lymphomas (including 4 of 7 immunoblastic lymphomas) were Epstein Barr virus positive.
Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas. As described in the text and Table 1, sections are presented from three cases: Case 1 (A–C); Case 2 (D–F); and Case 3 (G–I). A, D, G, hematoxylin and eosin stain reveals the cytological features of neoplastic cells. B, E, H, staining with a rat monoclonal antibody for the Kaposi's sarcoma–associated herpesvirus latency-associated nuclear antigen demonstrates nuclear staining in all neoplastic cells. C, F, I, staining with a mouse monoclonal antibody for Kaposi's sarcoma–associated herpesvirus viral interleukin-6 shows strong cytoplasmic staining in a minority of neoplastic cells. Original magnification in all panels, 500×.
We now describe the three Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas in more detail (Cases 1–3, Table 1). In Case 1, a 48-year-old white male developed Kaposi's sarcoma and nodal immunoblastic lymphoma simultaneously. Immunoblastic lymphoma cells co-expressed CD20 and CD3 and were Epstein Barr virus positive. Of interest, an adjacent lymph node had Kaposi's sarcoma and showed features of multicentric Castleman's disease, with scattered Kaposi's sarcoma–associated herpesvirus–positive plasmablasts in interfollicular areas. In Case 2, lymphoma presented as a soft-tissue neck mass in a 48-year-old white homosexual male, and Kaposi's sarcoma was diagnosed 8 months later. The immunoblastic lymphoma was both CD20 and CD3 negative and Epstein Barr virus positive. In Case 3, a 41-year-old white homosexual male (also a former injection drug user) developed idiopathic thrombocytopenic purpura and splenomegaly. This occurred when his CD4 lymphocyte count was 94 cells/mm3 and 1 month after Kaposi's sarcoma diagnosis. The spleen, on removal, showed extensive infiltration of the red pulp and large areas of tumor necrosis. Only small foci of viable tumor cells were identified. The tumor cells were CD20 and CD3 negative, λ restricted, and Epstein Barr virus negative.
Additionally, 5–20% of neoplastic cells in each Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphoma demonstrated positive cytoplasmic staining for Kaposi's sarcoma–associated herpesvirus viral interleukin-6 (Fig. 1)
DISCUSSION
We describe Kaposi's sarcoma–associated herpesvirus involvement in three immunoblastic lymphomas (43% of evaluable cases) arising in individuals with AIDS-associated Kaposi's sarcoma. Although primary effusion lymphoma can present first as a solid mass or subsequently develop an extracavitary component (1), our three Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas would not be classified as primary effusion lymphomas because they were not known to involve body cavities during their course (12). Still, these cases demonstrate features frequently found in primary effusion lymphoma and other recently described Kaposi's sarcoma–associated herpesvirus–positive non-Hodgkin's lymphomas (1, 4, 10). First, they occurred in persons who already had or subsequently developed Kaposi's sarcoma or multicentric Castleman's disease (1, 4). Second, they had an immunoblastic morphology along with an aberrant immunophenotype. Although some Kaposi's sarcoma–associated herpesvirus-associated lymphomas arising in multicentric Castleman's disease are described as “plasmablastic,” the distinction between immunoblastic lymphoma, the plasmablastic variant of diffuse large B-cell lymphoma, and primary effusion lymphoma is difficult, because most cases show overlapping histological features. Plasmacytoid differentiation and nuclear pleomorphism are generally prominent in AIDS-associated immunoblastic lymphomas, and plasmacytoid differentiation is often seen in Kaposi's sarcoma–associated herpesvirus-associated lymphoid proliferations (14). Third, two Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas were coinfected with Epstein Barr virus, similar to the profile of primary effusion lymphoma (1). The other Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphoma showed λ restriction, like multicentric Castleman's disease-associated plasmablastic lymphoma (3), and involved the spleen, which is also sometimes seen in multicentric Castleman's disease-associated non-Hodgkin's lymphoma (3, 4). The extensive infiltration of the red pulp with large areas of tumor necrosis favored a diagnosis of immunoblastic lymphoma over multicentric Castleman's disease in this case.
Localization of Kaposi's sarcoma–associated herpesvirus latency-associated nuclear antigen in the nuclei of all tumor cells of Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas provides evidence that Kaposi's sarcoma–associated herpesvirus was involved in the pathogenesis of these lymphomas. Prior systematic studies of immunoblastic lymphomas in HIV-infected persons relied exclusively on polymerase chain reaction to detect Kaposi's sarcoma–associated herpesvirus (2, 7, 8, 9). In those studies, given that Kaposi's sarcoma–associated herpesvirus was absent or present at low levels in a few cases, infection of tumor cells and infection of rare normal bystander lymphocytes could not be distinguished.
It is noteworthy that we found viral interleukin-6, a Kaposi's sarcoma–associated herpesvirus cytokine homologue, in a minority of tumor cells in each Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphoma. Viral interleukin-6, which is expressed during lytic viral replication, is also consistently detected in primary effusion lymphoma (in a minority of tumor cells) and multicentric Castleman's disease (in mantle zone plasmablasts; 15, 16). Viral interleukin-6 is secreted by primary effusion lymphoma cells and by Kaposi's sarcoma–associated herpesvirus-infected primary effusion lymphoma cell lines (13, 17). Furthermore, viral interleukin-6 receptors are present on primary effusion lymphoma cells, and viral interleukin-6 functions as an autocrine growth factor (13, 17). Therefore, intralesionally produced viral interleukin-6 could stimulate tumor cell growth in primary effusion lymphoma, multicentric Castleman's disease, and the Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas that we observed. Other Kaposi's sarcoma–associated herpesvirus gene products interact with cell-cycle control proteins, including p53, and might also play a role (18, 19).
There are two possible reasons that our study identified a higher fraction of Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas than did previous studies (2, 7, 8, 9). First, all of our subjects demonstrated poor immune-mediated control of Kaposi's sarcoma–associated herpesvirus infection, because they all also had Kaposi's sarcoma, and this may have predisposed them to Kaposi's sarcoma–associated herpesvirus-associated immunoblastic lymphoma. Second, because we selected for study only individuals known to be infected with Kaposi's sarcoma–associated herpesvirus (i.e., as manifested by Kaposi's sarcoma), the fraction of Kaposi's sarcoma–associated herpesvirus–associated immunoblastic lymphoma may have been especially high. This fraction, termed the population attributable risk (PAR), is calculated as follows:

where pKSHV is the prevalence of Kaposi's sarcoma–associated herpesvirus infection in the source population and RR is the relative risk for immunoblastic lymphoma associated with Kaposi's sarcoma–associated herpesvirus infection. We previously estimated this relative risk as 1.58 (11). When pKSHV = 1.00 (i.e., all subjects are infected with Kaposi's sarcoma–associated herpesvirus), the PAR is 37%, similar to what we observed for the fraction of Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas (43%). When pKSHV = 0.20 (i.e., roughly the Kaposi's sarcoma–associated herpesvirus prevalence among unselected HIV-infected persons), the PAR is only 10%. Thus, differences in the prevalence of Kaposi's sarcoma–associated herpesvirus in the source population may suffice to explain differences in the proportion of Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas in various reports.
A limitation of our study was the small number of cases for separate non-Hodgkin's lymphoma subtypes. Also, we are uncertain whether the lymphoma cases retrieved from pathology archives were fully representative of cases arising in conjunction with AIDS-associated Kaposi's sarcoma. As a result, it is difficult to generalize our findings to estimate the incidence of Kaposi's sarcoma–associated herpesvirus-associated non-Hodgkin's lymphomas. Furthermore, many specimens were obtained at autopsy, which limited the amount of available clinical data and made histologic characterization difficult. Although Kaposi's sarcoma–associated herpesvirus has been reported in non-Hodgkin's lymphomas associated with multicentric Castleman's disease (3, 4), we were unable to characterize how common it was for persons in our study to have this condition.
We conclude that Kaposi's sarcoma–associated herpesvirus is present in some immunoblastic lymphomas in persons with AIDS-associated Kaposi's sarcoma. Our immunoblastic lymphoma cases add to the spectrum of lymphomas associated with Kaposi's sarcoma–associated herpesvirus. Future work should characterize the incidence of Kaposi's sarcoma–associated herpesvirus–positive immunoblastic lymphomas in persons with HIV/AIDS and elucidate the role that Kaposi's sarcoma–associated herpesvirus plays in their genesis.
References
Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Said J, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 1996; 88: 645–56.
Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM . Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995; 332: 1186–91.
Dupin N, Diss TL, Kellam P, Tulliez M, Du M-Q, Sicard D, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 2000; 95: 1406–12.
Oksenhendler E, Boulanger E, Galicier L, Du M-Q, Dupin N, Diss TC, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002; 99: 2331–6.
Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA . Pleural and peritoneal lymphoma among people with AIDS in the United States. J Acquir Immune Defic Syndr 2002; 29: 418–21.
Oksenhendler E, Duarte M, Soulier J, Cacoub P, Welker Y, Cadranel J, et al. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. AIDS 1996; 10: 61–7.
Gessain A, Brière J, Angelin-Duclos C, Valensi F, Merle Béral H, Davi F, et al. Human herpes virus 8 (Kaposi's sarcoma herpes virus) and malignant lymphoproliferations in France: a molecular study of 250 cases including two AIDS-associated body cavity based lymphomas. Leukemia 1997; 11: 266–72.
Otsuki T, Kumar S, Ensoli B, Kingma DW, Yano T, Stetler-Stevenson M, et al. Detection of HHV-8/KSHV DNA sequences in AIDS-associated extranodal lymphoid malignancies. Leukemia 1996; 10: 1358–62.
Boye Hansen P, Penkowa M, Kirk O, Skinhøj P, Pedersen C, Lisse I, et al. Human immunodeficiency virus-associated malignant lymphoma in eastern Denmark diagnosed from 1990 to 1996: clinical features, histopathology, and association with Epstein-Barr virus and human herpesvirus-8. Eur J Haematol 2000; 64: 368–75.
Chadburn A, Cesarman E, Hyjek E, Lieu YF, Mulligan L, Said J, et al. KSHV-positive extra-cavitary lymphomas (EC-PELs) are part of the spectrum of primary effusion lymphomas (PEL) [abstract]. In: Sixth International Conference on Malignancies in AIDS and Other Immunodeficiencies, Bethesda, MD; 2002.
Engels EA, Rosenberg PS, Frisch M, Goedert JJ . Cancers associated with Kaposi's sarcoma (KS) in AIDS: a link between KS herpesvirus and immunoblastic lymphoma. Br J Cancer 2001; 85: 1298–303.
Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001.
Aoki Y, Yarchoan R, Braun J, Iwamoto A, Tosato G . Viral and cellular cytokines in AIDS-related malignant lymphomatous effusions. Blood 2000; 96: 1599–601.
Jaffe ES . Primary body cavity-based AIDS-related lymphomas. Evolution of a new disease entity. Am J Clin Pathol 1996; 105: 141–3.
Katano H, Sato Y, Kurata T, Mori S, Sata T . Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology 2000; 269: 335–44.
Parravicini C, Chandran B, Corbellino M, Berti E, Paulli M, Moore PS, et al. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am J Pathol 2000; 156: 743–9.
Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G . Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 1999; 94: 2871–9.
Friborg J Jr, Kong W, Hottiger MO, Nabel GJ . p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 1999; 402: 889–94.
Rivas C, Thlick A-E, Parravicini C, Moore PS, Chang Y . Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J Virol 2001; 75: 429–38.
Acknowledgements
The authors thank the collaborating institutions and individuals of the National Cancer Institute's AIDS Cancer Specimen Bank for providing the specimens used in this study, in particular Debra Garcia (University of California, San Francisco), who coordinated access to specimens. We also thank Steven Miles and Jonathan Said (University of California, Los Angeles) for providing additional specimens. We gratefully acknowledge the laboratory contributions of Giovanna Tosato (National Cancer Institute). Finally, we thank the National Cancer Institute Pathology Department's immunohistochemistry laboratory, particularly Leong Sonn Mann, for assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engels, E., Pittaluga, S., Whitby, D. et al. Immunoblastic Lymphoma in Persons with AIDS-Associated Kaposi's Sarcoma: a Role for Kaposi's Sarcoma–Associated Herpesvirus. Mod Pathol 16, 424–429 (2003). https://doi.org/10.1097/01.MP.0000056629.62148.55
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000056629.62148.55
This article is cited by
-
HHV8-related lymphoid proliferations: a broad spectrum of lesions from reactive lymphoid hyperplasia to overt lymphoma
Modern Pathology (2017)
-
Human herpesvirus 8 – A novel human pathogen
Virology Journal (2005)
-
High incidence of Kaposi sarcoma-associated herpesvirus infection in HIV-related solid immunoblastic/plasmablastic diffuse large B-cell lymphoma
Leukemia (2005)