Abstract
Insulin-like growth factor-II messenger RNA (mRNA)-binding protein-3 (IMP-3), also known as K homology domain-containing protein overexpressed in cancer (KOC) and L523S, is a member of the insulin-like growth factor-II mRNA-binding protein family and is expressed during embryogenesis and in some malignancies. IMP-3 expression in melanocytic neoplasms has not been investigated. Fifty-six melanocytic neoplasms from 48 subjects were immunohistochemically studied using a monoclonal antibody against L523S/IMP-3. IMP-3 expression in melanoma was significantly higher than in Spitz nevi (P<0.05), and the staining intensity in the Spitz nevi was weak. IMP-3 expression in metastatic melanoma was significantly higher than in primary cutaneous melanoma with a Breslow depth ≤1 mm (P<0.01). None of the benign nevi and dysplastic nevi expressed IMP-3. Our study demonstrates that IMP-3 is expressed in malignant melanoma but not in benign nevi, even when dysplastic features are present; IMP-3 is expressed in a significantly higher proportion of melanomas than Spitz nevi; and IMP-3 is expressed in metastatic melanomas significantly more than in thin melanomas. In conclusion, IMP-3 appears to be involved in the progression of malignant melanoma and may play an important role in the regulation of the biologic behavior of this tumor. Additionally, IMP-3 may have diagnostic utility in distinguishing melanoma from benign nevic cells, dysplastic nevi, and Spitz nevi.
Similar content being viewed by others
Main
Cutaneous melanocytic neoplasms are a diverse group of lesions that includes benign melanocytic nevi, nevi with architectural disorder and cytologic atypia (dysplastic nevi), Spitz nevi, and malignant melanoma. Although most histopathological diagnoses can be made with high specificity based exclusively upon morphologic criteria, a small proportion of cutaneous melanocytic lesions pose diagnostic difficulties. In particular, Spitz nevi and dysplastic nevi may be difficult to distinguish from melanoma.1, 2 While several immunohistochemical stains have been reported to be helpful adjuncts, in particular Ki-67 (MIB-1) for analyzing proliferation rate, higher in melanoma than Spitz nevi,3 there is a need for additional immunohistochemical tools to help differentiate between these difficult melanocytic lesions.
Another challenge is determining prognosis in patients with newly diagnosed melanoma. The most widely utilized prognostic factors are Breslow depth—a measurement of tumor thickness—and sentinel lymph node status.4 The model of tumor progression in malignant melanoma consists of a non-tumorigenic in situ radial growth phase, an indolent invasive radial growth phase, and a tumorigenic vertical growth phase.5, 6 Vertical growth phase, high mitotic rate, and regression are other factors that may increase the risk of recurrence or metastasis of melanoma.5, 7, 8 Sentinel lymph node biopsy is currently recommended for patients with melanomas that have a Breslow depth >1 mm or ≤1 mm when there is ulceration or Clark's level IV or V invasion.9 Both greater Breslow depth and metastatic melanoma in sentinel lymph nodes are associated with a worse prognosis.4, 10 Additionally, although most thin melanomas (Breslow thickness ≤1 mm) have a favorable prognosis, some thin melanomas are more aggressive; consequently, there is a need to identify thin melanomas with a more aggressive potential to guide management.4, 11, 12
Insulin-like growth factor-II (IGF-II) messenger RNA (mRNA)-binding protein-3 (IMP-3), also known as K homology domain-containing protein overexpressed in cancer (KOC) and L523S, is a member of the IGF-II mRNA-binding protein (IMP) family, which also includes IMP-1 and IMP-2.13 IMP-3 is a 580 amino-acid protein encoded by a 4350-bp mRNA transcript produced by a gene located on chromosome 7p11.5.14 As a translational activator of IGF-II leader-3 mRNA, it is associated with cell proliferation and is considered an oncofetal protein due to its expression during embryogenesis and in some malignancies.15 Normal adult tissues that express IMP-3 include term placenta, ovary, testis, brain, lymph node germinal centers, and intestinal mucosa.13, 16, 17 Increased levels of IMP-3 have been identified in pancreatic carcinoma, renal cell carcinoma, germ cell neoplasms, ovarian carcinoma, and extrapulmonary small-cell carcinoma, as well as high-grade neuroendocrine carcinoma, squamous cell carcinoma, and adenocarcinoma of the lung.16, 17, 18, 19, 20, 21 Additionally, IMP-3 was shown to be a prognostic marker in patients with renal cell carcinoma, with lack of expression in the primary tumor predicting longer metastases-free survival.22 IMP-3 expression increases with grade of dysplasia in pancreatic ductal epithelium and with tumor stage in pancreatic carcinoma.19 Finally, two studies demonstrating inhibition of cell proliferation after knockdown of IMP-3 in human K562 leukemia cells15 and an immunogenic response to administration of IMP-3 in lung cancer patients21 show promise for IMP-3 as a potential target molecule for cancer therapy.
These findings lead us to hypothesize that IMP-3 may be a tumor progression marker in malignant melanoma. We propose to test this hypothesis by analyzing the expression of IMP-3 in melanocytic neoplasms by immunohistochemistry.
Materials and methods
Case Selection
This study was conducted in accordance with a protocol approved by the institutional review board of the University of Rochester Medical Center. A total of 56 biopsied and surgically resected melanocytic neoplasm specimens from 48 subjects dating from June 2004 to January 2006 were retrieved from the surgical pathology archives at the University of Rochester Medical Center. The specimens included 11 benign nevi, 8 dysplastic nevi, 10 Spitz nevi, 17 primary cutaneous melanomas, and 10 metastatic melanomas. Matched primary and metastatic melanoma specimens were available for six subjects. All cases were diagnosed by a dermatopathologist (GS) using standard criteria. Primary cutaneous melanomas were subdivided into two groups: tumors with a Breslow depth ≤1 mm and tumors with a Breslow depth>1 mm. Hematoxylin and eosin-stained slides were re-examined to confirm the diagnosis, and representative sections were selected for immunohistochemistry.
Malignant Melanoma Staging
Pathologic and clinical stages for the 17 subjects with primary cutaneous melanoma were determined using the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 6th edition, staging system for melanoma of the skin.23 The subjects with primary cutaneous melanoma were subdivided into two groups: subjects with clinical stage I and II melanoma and subjects with clinical stage III and IV melanoma.
Immunohistochemical Analysis
Immunohistochemical studies were performed on 5-μm formalin-fixed, paraffin-embedded tissue sections using a mouse monoclonal antibody against L523S/IMP-3 (clone 69.1; Dako North America, Carpinteria, CA, USA). Tissue sections were deparaffinized according to established procedures and quenched with 3% H2O2 for 6 min. Sections were washed in running water and Tris-buffered saline (TBS) consisting of 50 mmol/l Tris-HCl (pH 7.6), 150 mmol/l NaCl, and 0.05% Tween 20. Antigen retrieval was performed using preheated (95–99°C) target retrieval solution, modified citrate buffer, pH 6.1 (Dako), in a Black and Decker steamer (Model HS800; Shelton, CT, USA) for 30 min, followed by a 15-min cool down period. Slides were rinsed with TBS for 5 min and mounted in the Dako Autostainer. Sections were incubated with the monoclonal mouse anti-L523S/IMP-3 antibody (dilution 1:100) at room temperature for 40–60 min. The sections were then incubated for 30 min with EnVision+ System horseradish peroxidase (HRP)-labeled polymer conjugated to goat anti-mouse (Dako). Staining was developed with 3-amino-9-ethylcarbazole (AEC)+substrate-chromogen (Dako) for 10 min. Slides were rinsed in running distilled water, counterstained with modified Mayer's hematoxylin, and blued in 0.3% ammonia water followed by a tap water rinse. Slides were mounted using an aqueous medium and viewed under a light microscope. Cytoplasmic staining was considered positive for IMP-3 expression. The percentage of tumor cells with cytoplasmic staining was recorded. The intensity of cytoplasmic staining was recorded as weak, moderate, or strong.
Statistical Analysis
Statistical analysis was carried out using the SAS system (SAS Institute Inc., Cary, NC, USA). P<0.05, as determined by two-tailed Fisher's exact test, was considered statistically significant.
Results
Clinicopathologic Features of Melanocytic Neoplasms
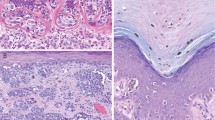
The clinical features of the study set are presented in Table 1. The 11 benign nevi were from 11 subjects (2 male and 9 female) whose ages ranged from 14 to 66 years (mean=44.5). Histologically, they were characterized by small nests of nevomelanocytic cells without cytological atypia; seven were dermal nevi (Figure 1a) and the remaining four were compound nevi. The eight dysplastic nevi were from eight subjects (4 male and 4 female) whose ages ranged from 21 to 68 years (mean=44.0). Histologically, they included both junctional and compound variants and ranged in severity of dysplasia from mild to severe; features included lentiginous hyperplasia of atypical melanocytes, irregular nests with occasional bridging, stromal fibrosis, and a mild host inflammatory response (Figure 1b). The 10 Spitz nevi were from 10 subjects (4 male and 6 female) whose ages ranged from 2 to 44 years (mean=16.3). Histologically, they were characterized by spindled to epithelioid melanocytes arranged in fascicles or nests with clefting and occasional intraepidermal spread of melanocytes over the central portion of the lesions (Figure 1c). The 17 primary cutaneous melanomas were from 17 subjects (9 male and 8 female) whose ages ranged from 3 to 81 years (mean=52.8). Histologically, they were characterized by asymmetric proliferations of highly atypical melanocytes associated with occasional pagetoid spread, mitotic activity, a prominent host inflammatory reaction, and pigmentary incontinence (Figure 1d). The primary cutaneous melanomas were all invasive and had Clark's levels ranging from II to V and Breslow depths ranging from 0.275 mm to 2 cm (Table 2); three primary melanomas were ulcerated, and four arose from a pre-existing nevus. The pathologic and clinical stages for the 17 subjects with primary cutaneous melanoma are presented in Table 2. The 10 metastatic melanomas were from 10 subjects (8 male and 2 female) whose ages ranged from 3 to 82 years (mean=47.8). Eight metastatic melanomas were localized to regional lymph nodes, one metastasis was from the lung, and one was from the soft tissue of the arm.
IMP-3 Expression by Immunohistochemistry
The results of immunohistochemical studies of IMP-3 expression in 56 melanocytic neoplasm specimens are summarized in Table 3. None of the benign nevi and dysplastic nevi expressed IMP-3. Of 10 Spitz nevi, two (20%) expressed IMP-3 in 50–90% of tumor cells (Figure 2a), and one (10%) expressed IMP-3 in 5–9.9% of tumor cells. The immunostaining intensity in the Spitz nevi that expressed IMP-3 was weak. Twenty-three of 27 (85%) melanomas expressed IMP-3, and the immunostaining intensity in the melanomas was moderate to strong (Figure 2b). After subdividing by tumor thickness, 4 of 6 (67%) primary cutaneous melanomas with a Breslow depth ≤1 mm expressed IMP-3 (Figure 2c), whereas 10 of 11 (91%) primary cutaneous melanomas with a Breslow depth >1 mm expressed IMP-3 (Figure 2d). After subdividing by clinical stage, 8 of 10 (80%) tumors from subjects with clinical stage I or II melanoma expressed IMP-3, whereas 6 of 7 (86%) tumors from subjects with clinical stage III or IV expressed IMP-3. The percentage of melanocytic neoplasms expressing IMP-3 in 10% or more of tumor cells is presented in Table 3. Significantly more melanomas expressed IMP-3 in 10% or more of tumor cells than Spitz nevi (P=0.0174), and significantly more metastatic melanomas expressed IMP-3 in 10% or more of tumor cells than primary cutaneous melanomas with a Breslow depth ≤1 mm (P=0.0075).
IMP-3 immunohistochemistry showing (a) weak cytoplasmic expression in the dermal component of a Spitz nevus and (b) strong cytoplasmic expression in metastatic melanoma. Compared to (c) patchy IMP-3 expression in a malignant melanoma with a Breslow depth ≤1 mm, (d) malignant melanoma with a Breslow depth >1 mm expresses IMP-3 in >90% of tumor cells. Note the IMP-3 expression in the hair follicle in part (c) (original magnification × 400: a, b; × 200: c, d).
Discussion
Our study demonstrates that IMP-3 is expressed in malignant melanoma but not in benign melanocytic nevi—even when dysplastic features are present. IMP-3 is expressed in a significantly higher proportion of melanomas than Spitz nevi, and it is expressed in significantly more metastatic melanomas than thin melanomas (Breslow depth ≤1 mm). As a member of the IGF-II mRNA-binding protein family, IMP-3 is involved in the regulation of cell proliferation and appears to play a role in the tumorigenesis and biologic behavior of a number of malignancies.13, 15 In view of our observations, IMP-3 represents a novel marker of progression in melanoma and may have an important role in the regulation of biologic behavior in this tumor.
Immunohistochemical detection of IMP-3 expression may facilitate the histopathological distinction between dysplastic nevi and melanoma, and between Spitz nevi and melanoma. IMP-3 was expressed in a significantly higher proportion of melanomas than benign nevi (P=0.0003), dysplastic nevi (P=0.0003), and Spitz nevi (P=0.0215). However, the utility of IMP-3 in the differentiation of melanomas from Spitz nevi and dysplastic nevi may be limited due to the fact that IMP-3 expression was low in primary cutaneous melanomas with a Breslow depth ≤1 mm. IMP-3 may prove to be more useful diagnostically in differentiating benign nevic rests from metastatic melanoma in sentinel lymph nodes; additional studies in this area are needed. Also of potential diagnostic utility was our finding that only melanoma demonstrated strong immunohistochemical expression of IMP-3; strong expression of IMP-3 as detected by immunohistochemistry thus favors a diagnosis of melanoma when the differential diagnosis of a melanocytic tumor includes Spitz nevus.
Although our results suggest that IMP-3 may represent a prognostic marker in melanoma, our study did not include long-term survival data. Consequently, although we were able to demonstrate that IMP-3 is expressed significantly more in metastatic melanomas than thin melanomas, we were not able to show that patients with thin melanomas that express IMP-3 have more aggressive tumors. A larger study comparing IMP-3 expression with long-term survival data will be needed to determine whether IMP-3 might be used at initial diagnosis to identify patients who may benefit from more careful monitoring or aggressive clinical management. Furthermore, although our study suggests that IMP-3 expression increases with greater Breslow depth and clinical stage, our results did not attain statistical significance—most likely due to the small sample size. On the basis of our results, additional studies correlating IMP-3 expression with Breslow depth and clinical stage are warranted.
In conclusion, IMP-3 is a novel progression marker in malignant melanoma and may have diagnostic utility in distinguishing melanoma from benign nevic cells, dysplastic nevi, and Spitz nevi. IMP-3 also represents a potential therapeutic target for new modalities in the treatment of malignant melanoma.
References
Bron JL, Jaspars EH, Molenkamp BG, et al. Three patients with a Spitz naevus that later turned out to be a melanoma. Ned Tijdschr Geneeskd 2005;149:1852–1858.
Troxel D . Medicolegal aspects of error in pathology. Arch Pathol Lab Med 2006;130:617–619.
Bergman R, Malkin L, Sabo E, et al. MIB-1 monoclonal antibody to determine proliferative activity of Ki-67 antigen as an adjunct to the histopathologic differential diagnosis of Spitz nevi. J Am Acad Dermatol 2001;44:500–504.
Garbe C, Eigentler TK . Diagnosis and treatment of cutaneous melanoma: state of the art 2006. Melanoma Res 2007;17:117–127.
Clark Jr WH, Elder DE, Guerry DIV, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 1989;81:1893–1904.
Guerry D, Synnestvedt M, Elder DE, et al. Lessons from tumor progression: the invasive radial growth phase of melanoma is common, incapable of metastasis, and indolent. J Invest Dermatol 1993;100:342S–345S.
Oliveira Filho RS, Ferreira LM, Biasi LJ, et al. Vertical growth phase and positive sentinel node in thin melanoma. Braz J Med Biol Res 2003;36:347–350.
Bedrosian I, Faries MB, Guerry DT, et al. Incidence of sentinel node metastasis in patients with thin primary melanoma (< or =1 mm) with vertical growth phase. Ann Surg Oncol 2000;7:262–267.
Cecchi R, Buralli L, Innocenti S, et al. Sentinel lymph node biopsy in patients with thin melanomas. J Dermatol 2007;34:512–515.
Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med 2006;355:1307–1317.
Ramsden AJ, Grover R, Chana J, et al. A prospective analysis of c-myc oncoprotein levels as a prognostic marker in malignant melanoma. J Plast Reconstr Aesthet Surg 2007;60:626–630.
Gimotty PA, Elder DE, Fraker DL, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol 2007;25:1129–1134.
Nielsen J, Christiansen J, Lykke-Andersen J, et al. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol 1999;19:1262–1270.
Müeller-Pillasch UL, Wallrapp C, Micha A, et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 1997;14:2729–2733.
Liao B, Hu Y, Herrick DJ, et al. The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J Biol Chem 2005;280:18517–18524.
Hammer NA, Hansen TvO, Byskov AG, et al. Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction 2005;130:203–212.
Simon R, Bourne PA, Yang Q, et al. Extrapulmonary small cell carcinomas express K homology domain containing protein overexpressed in cancer, but carcinoid tumors do not. Hum Pathol 2007;38:1178–1183.
Gu L, Shigemasa K, Ohama K . Increased expression of IGF II mRNA-binding protein 1 mRNA is associated with an advanced clinical stage and poor prognosis in patients with ovarian cancer. Int J Oncol 2004;24:671–678.
Yantiss RK, Woda BA, Fanger GR, et al. KOC (K homology domain containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol 2005;29:188–195.
Xu H, Bourne PA, Spaulding BO, et al. High-grade neuroendocrine carcinomas of the lung express K homology domain containing protein overexpressed in cancer but carcinoid tumors do not. Hum Pathol 2007;38:555–563.
Wang T, Fan L, Watanabe Y, et al. L523S, an RNA-binding protein as a potential therapeutic target for lung cancer. Br J Cancer 2003;88:887–894.
Jiang Z, Chu PG, Woda BA, et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol 2006;7:556–564.
Greene F, Page D, Fleming I, Fritz A, Balch C, Haller D, Morrow M (eds). AJCC Cancer Staging Manual, 6th edn. Springer-Verlag: Berlin/Heidelberg/New York/London/Paris/Tokyo/Hong Kong, 2002. 435pp.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
The authors state that they have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Pryor, J., Bourne, P., Yang, Q. et al. IMP-3 is a novel progression marker in malignant melanoma. Mod Pathol 21, 431–437 (2008). https://doi.org/10.1038/modpathol.3801016
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3801016
Keywords
This article is cited by
-
Immunohistochemical staining for IMP3 in patients with duodenal papilla tumors: assessment of the potential for diagnosing endoscopic resectability and predicting prognosis
BMC Gastroenterology (2021)
-
The biological function of IGF2BPs and their role in tumorigenesis
Investigational New Drugs (2021)
-
Usefulness of IMP3 and FOXP3 to predict metastasis of cutaneous melanomas
Surgical and Experimental Pathology (2018)
-
IGF2BP3 functions as a potential oncogene and is a crucial target of miR-34a in gastric carcinogenesis
Molecular Cancer (2017)
-
The involvement of insulin-like growth factor 2 binding protein 3 (IMP3) in pancreatic cancer cell migration, invasion, and adhesion
BMC Cancer (2015)