Abstract
The ovarian epithelial cells carrying heterozygous BRCA1 or BRCA2 mutation (we called BRCA1/2 mutation) are known to predispose to the development of ovarian cancer; however, the molecular basis of such predisposition is largely unknown. We hypothesize that BTAK may be a potential target for heterozygous BRCA1/2 mutation. We sought to determine the relationship between the status of BRCA1/2 heterozygous mutation and BTAK expression in prophylactically removed ovaries as compared with normal ovaries and ovarian cancer controls. Western blot analysis of BTAK was performed in a primary cell culture carrying heterozygous BRCA1 mutation and three normal ovarian surface epithelial cell cultures. Immunohistochemical analysis of BTAK expression was also performed by image analysis in ovaries of 21 patients with known BRCA1/2 mutation or very strong family history of breast/ovarian cancer that underwent prophylactic oophorectomy, 38 normal ovaries from patients without any known mutation, and 194 ovarian carcinomas. The BTAK expression was significantly increased in primary culture carrying a heterozygous BRCA1 mutation as compared to those with no known BRCA1/2 mutation. Immunohistochemical staining of BTAK showed increased expression in ovarian epithelial cells carrying BRCA1/2 mutation or strong breast/ovarian family history compared with normal ovaries (P<0.001). Higher BTAK expression was found in ovarian cancer cells compared to ovaries without cancer but with known BRCA1/2 mutation or strong family history (P<0.001), and expression levels of BTAK and p53 were directly correlated (r=0.306; P<0.001). Increased expression of BTAK is directly correlated with mutation status of BRCA1/2 genes, suggesting that mutation in a single allele of either BRCA1 or 2 may be responsible for the activation of BTAK. This activation may be a key early genetic event in the development of hereditary ovarian cancer.
Similar content being viewed by others
Main
BRCA, breast cancer susceptibility gene, plays an important role in maintaining genomic stability and acts as a tumor suppressor. Mutations of BRCA1 and BRCA2 genes are associated with increased susceptibility for breast and ovarian cancer. These mutations increase the risk for developing ovarian cancer to 26% (BRCA1) and 10% (BRCA2) during a woman's lifetime.1 Since the cloning of both genes more than a decade ago, enormous amount of work has been done to elucidate their functions. It has been shown that both BRCA1 and 2 are involved in multiple cellular pathways.2, 3 BRCA1 can arrest cell cycle progression by stimulating the transcription of the cyclin-dependent kinase (CDK) inhibitor p21WAF/Cip1.4 BRCA1 also binds and works cooperatively with p53 in vivo;5 p53, in turn, modulates BRCA1 expression.6 BRCA-deficient cells display spontaneous chromosomal abnormalities, defective G2/M transit, centrosome amplification, and defects in both homologous DNA recombination and transcription-coupled base-excision repair of oxidative DNA damage.7, 8 Cells with defective BRCA1 are hypersensitive to DNA-damaging agents, are slower to repair double-stranded DNA breaks, and show impairment in transcription-coupled repair.7 BRCA1 can also promote homologous recombination, and has been to shown to interact with other proteins required for DNA end-joining, nucleotide mismatch repair, DNA replication, homologous recombination repair, and signal transduction in response to damage. BRCA2 repairs the double-strand break during the S phase of the cell cycle and also participates in cytokinesis. The abnormalities of chromosome number seen in BRCA2-deficient cells are a direct consequence of BRCA2 dysfunction. Moreover, BRCA2 role in cytokinesis provides a mechanism for the generation of polyploidy.9 All of this suggests that BRCA may have multiple functions in the maintenance of genetic stability and cell cycle progression. However, one unsolved puzzle is how the heterozygous BRCA mutation in histologically normal ovarian surface epithelial cells predisposes to cancer development in the ovary, particularly whether or not these cells exhibit the haplotype insufficiency.10, 11, 12
One common characteristic of human cancers is genetic instability, which expresses in numerical and structural abnormalities of the chromosomes. During cell division, centrosomes play an important role in the equal separation of the chromosomes. BTAK, encoding a centrosome-associated kinase, regulates the mitotic progression.13, 14 This kinase, with synonyms such as Aurora-2, Aurora-A, ARK1, STK15, is involved in the chromosomal segregation and centrosome translocation to the mitotic spindle in the early mitotic phase. Overexpression of BTAK also results in defective spindle assembly checkpoint, allowing cells with abnormal chromosomal separation to enter anaphase, leading to aneuploidy.15 Recent studies have demonstrated that activation of BTAK is required for mitotic entry, centrosome maturation and separation, and G2 to M transition.16, 17 Human BTAK is amplified and overexpressed in various carcinomas such as breast, colorectal, gastric, pancreatic tumors as well as ovarian, bladder, and esophageal cancers.18, 19, 20, 21 These studies indicate that BTAK may play a key role in the pathogenesis of human cancers through the maintenance of genetic stability.
Recent studies have suggested that BTAK physically binds and phosphorylates BRCA impairing the regulation of the G2/M transition.22 Studies from BRCA1 knockout mice have demonstrated that BRCA is essential in centrosome localization and duplication, which suggests BRCA plays an important role in mitosis,23, 24 thus BRCA1 and BTAK involve similar cellular processes. We hypothesize that BRCA1 and BTAK interactions play a critical role in ovarian tumorigenesis and such interaction occurs at a very early stage and even in primary cells carrying a heterozygous mutation. To test this hypothesis, we examined the expression of BTAK in ovaries from 21 patients with known BRCA1/2 mutation, 38 normal ovaries, and 194 ovarian cancers with the purpose of establishing epidemiological evidence that BTAK may be one of early targets activated by BRCA heterozygous mutation.
Materials and methods
Western Blot Analysis
The primary human ovarian epithelial cells OSE72, OSE103, OSE137, and OSE76 were cultured with complete medium containing 15% fetal bovine serum (FBS) described previously.25 Cells (OSE72, OSE103, OSE137, and OSE76) were washed twice with phosphate-buffered saline (PBS) and homogenized in lysis buffer (150 mM NaCl, 50 mM HEPES (pH 7.2), 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1% Tween 20, 0.1 mM phenylmethylsulfonylfluoride, 2.5 μg/ml leupeptin, 0.1 mM sodium pyrthovanadate) (Sigma Chemicals, St Louis, MO, USA) at 4°C for 30 min. Lysates were then spun at 10 000 g for 10 min at 4°C. The resulting supernatant was transferred to a clean tube and the total protein concentration was determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories). The samples were then boiled in polyacrylamide gel sample buffer containing SDS (SDS sample buffer) for 5 min. Equal amounts (50 μg) of total protein was separated by 12% SDS-PAGE gels and transferred to Hybond-C nitrocellulose membranes (Amersham Life Science) by electroblotting. The membranes were blocked in 5% solution (TBS-T containing skim milk) overnight and then sequentially incubated with the affinity-purified rabbit polyclonal BTAK antibody (Gene Tex; 1:1000, San Antonio, TX, USA) as primary antibody and horseradish-peroxidase-conjugated goat anti-rabbit IgG as a secondary antibody (NA 934V, Amersham Pharmacia) and visualized by using the electrochemiluminescence (ECL) (Amersham Pharmacia Biotech) detection system. To confirm equivalent loading of total protein in all lanes, the membranes were reprobed with β-actin antibody.
Tissue Sample
Ovaries from 21 patients that underwent prophylactic oophorectomy (University of Colorado Health Sciences Center) for known BRCA1 (5 cases) or BRCA2 mutation (6 cases) or strong personal and family history of breast/ovarian cancer (10 cases) were included in this study. Pathology of oophorectomy specimen was reviewed by single pathologist (MS). In addition, 38 normal ovaries and 194 cases of ovarian high-grade serous carcinomas were also studied (University of Texas MD Anderson Cancer Center). Normal ovaries and ovarian carcinoma cases were selected from cases that had undergone initial surgery at the University of Texas MD Anderson Cancer Center between 1990 and 2000. The use of tissue followed IRB-approved protocol. Tissue microarray construction was performed as previously described.26 Cases in which no tumor was found or no epithelial cells were present were excluded from the final data analysis.
Immunohistochemical Analysis
The tissue slides were subjected to immunohistochemical staining as follows. After initial deparaffinization, endogenous peroxidase activity was blocked by using 0.3% hydrogen peroxide. Deparaffinized sections were microwaved in 10 mM citrate buffer (pH 6.0) to unmask the epitopes. The slides were then incubated against BTAK (Gene Tex; 1:100, San Antonio, TX, USA) and p53 (Santa Cruz; 1:1000, Santa Cruz, CA, USA) for 1 h at ambient temperature. Next, the slides were incubated with biotin-labeled secondary antibody for 20 min, and finally with a 1:40 solution of streptavidin:peroxidase for 20 min. Tissues were then stained for 3 min with freshly prepared 0.05% 3′, 3-diaminobenzidine tetrahydrochloride in 0.05 M Tris buffer at pH 7.6 containing 0.024% H2O2 and then counterstained with hematoxylin, dehydrated, and mounted. All of the dilutions of antibody, biotin-labeled secondary antibody, and streptavidin-peroxidase were made in PBS (pH 7.4) containing 1% bovine serum albumin.
Quantitation of Immunohistochemistry
The BTAK intensity of staining was analyzed by computerized image analysis (Ariol SL-50, Applied imaging, San Jose, CA, USA). Quatitation is carried out by measuring all the pixels in a digitalized image and calculating the average. A black pixel has a value of 0 and a white pixel a value of 255. Therefore, a dark staining will have a low value (close to 0) and a light staining a higher value (close to 255). The mean relative optical density was expressed as arbitrary units of intensity and used for analysis. For statistical purposes, optical density values were grouped in a 4-score grading system as the mean optical density±s.d. (177.46±15.10). Absence of staining was defined as negative and given a score of 0 (the optical density≥190), weak expression a score of (optical density≥180 and <190), moderate expression a score of 2 (the optical density≥170 and <180), and strong expression a score of 3 (optical density<170). Evaluation of the average expression for p53 expression was performed visually by two pathologists (ZZ and DGR), independently in a blinded manner as follows: 0, less than 10% nuclear staining; 1, more than 10%, less than 25% nuclear staining; 2, more than 25%, less than 50% nuclear staining; 3, more than 50% nuclear staining, discrepancy was resolved by third pathologist (JL).
Statistical Analysis
Differences in proportions were evaluated by the χ2 analyses. Kruskal–Wallis and Mann–Whitney test were used to compare multiple independent samples containing normal ovaries, mutation ovaries, and ovarian carcinomas. The relationship between expression of BTAK and p53 was analyzed with Spearman Rank Order Correlation test. The basic descriptive statistical analysis and tables were created using the Statistica software package, version 6.0 (Statsoft, Tulsa, OK, USA). Results were considered statistically significant at the P<0.05 level.
Results
Expression of BTAK in Primary Ovarian Epithelial Cell Cultures with or without BRCA1 Heterozygous Mutation
BTAK expression was examined by western blot analysis in four primary ovarian epithelial cell cultures, three of which without known BRCA mutation (OSE72, OSE103, and OSE137) and one with a known BRCA1 mutation (OSE76). A specific band of BTAK protein (46 kDa) was detected strongly in OSE76 cell line by western blot, but no clear bands were found in OSE72, OSE103, and OSE137 (Figure 1), suggesting that BTAK expression was increased in primary ovarian epithelial cells carrying a BRCA1 mutation.
Comparison of BTAK and P53 Expression in Normal Ovaries, Ovaries with BRCA Mutations or Strong Family History of Breast/Ovarian Cancer and Ovarian Carcinomas
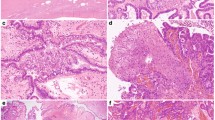
Next, we performed immunohistochemical staining of morphologically normal ovaries in a cohort of 21 patients who had prophylactic surgery and compared these with 38 normal ovaries and 194 ovarian cancer controls. Results are shown in Table 1. The expression of BTAK showed 1+ positivity in eight of 21 cases, 2+ positivity in seven of 21 cases, and 3+ positivity in one of 21 cases in ovaries carrying a heterozygous BRCA mutation as compared with 38 normal control ovaries, which showed 1+ positivity in seven of 38 cases (18%), 2+ positivity in two of 38 (5%), and no 3+ positivity in any of the normal ovaries (P<0.001; Mann–Whitney test), while ovarian cancer showed further increase in BTAK expression in all 194 cases as compared with BRCA heterozygous ovaries (P<0.001; Mann–Whitney test). The expression of p53 showed moderate to strong positivity in two of 38 cases (6%) in normal ovary, weakly increased expression in seven of 21 cases (33%) with known BRCA mutation or strong family history. p53 is highly expressed in 116 of 194 (60%) of ovarian carcinomas. Using Spearman correlation rank order test, we observed BTAK was correlated with p53 (r=0.306, P<0.001). The summary of BTAK expression is shown in Figure 2. Representative pictures of immunohistochemical staining for BTAK and p53 in normal ovary, prophylactic ovary and ovarian cancer are shown in Figure 3.
Immunohistochemical staining for BTAK and p53 protein expression. (a) No expression of BTAK was found in normal ovarian surface epithelial cells. (b) No expression of p53 was found in normal ovarian surface epithelial cells. (c) Cytoplasmic expression of BTAK was found in the ovarian epithelial cells carrying BRCA mutation. (d) Nuclear staining of p53 was found in the ovarian epithelial cells carrying BRCA mutation. (e) Strong cytoplasmic staining of BTAK was found in the OCA. (f) Strong nuclear staining of p53 was found in the OCA. Sections were counterstained with hematoxylin. Original magnification × 400.
Discussion
One of major unresolved issues in the field is whether BRCA heterozygous mutation carriers exhibit different phenotype from non-mutational carriers. While several studies have failed to detect such difference at the histopathological level,11, 27, 28, 29, 30 others have detected such differences between these two different groups.10, 12, 31, 32, 33, 34, 35, 36, 37 In addition, human ovarian-surface epithelial (HOSE) cells from patients with a family history of breast or ovarian cancer show increased CA-125 expression34 and more stable expression of the Met receptor for hepatocyte growth factor (HGF) than do HOSE cells from patients without such a history.38 Transformation of HOSE cells with the SV40 T/t antigen led to increased telomere instability and reduced growth potential in cells from patients with a family history of breast or ovarian cancer, indicating that those cells were closed to replicative senescence.39 Furthermore, He40 found that several genes involved in protein synthesis are upregulated in ovarian surface epithelial cells derived from prophylactic oophorectomies but not in those without BRCA mutations. All of these findings suggest that ovarian epithelial cells with heterozygous BRCA1 or BRCA2 germline mutations are biologically different from cells without such germline mutations, either through haplo-insufficiency or through predisposition to a loss of the second wild-type allele of the BRCA1 gene. However, the differences between these two groups are relatively subtle as reflected in the existing conflicting literatures on this topic. The results described here support a latter view: HOSE cells carrying heterozygous mutations are different from those without mutations, and BTAK is a potential target that is activated by a heterozygous BRCA1 or BRCA2 mutation in morphologically normal ovarian surface epithelial cells.
Human BTAK gene is located in the 20q13 chromosome region and is involved in the G2–M checkpoint and mitosis commitment.41 It is amplified and overexpressed in several different types of malignant tumors including ovarian carcinomas.42, 43 Recent studies reported that BRCA is also localized in the centrosome 23, 44 and BTAK and BRCA1 form a complex.22 Ouchi et al22 showed recently that BTAK physically binds and phosphorylates BRCA1, and the phosphorylation is correlated with impaired function of BRCA1 in regulating G2/M transition. This suggests a link between BTAK expression and impaired BRCA1 function in genetic instability and tumorigenesis. Our results suggest that BTAK is overexpressed in prophylactic ovaries with known BRCA mutation. Biochemical evidence showed that BTAK and BRCA form a complex in vivo and BRCA1 is the substrate of BTAK. Mutation in one of two alleles of BRCA may decrease the physiological substrate for BTAK and lead to further increase in BTAK expression.45 Such increased BTAK activity may lead to genetic instability and p53 inactivation, which can lead to initiation of transformation of ovarian epithelial cells. Our results are consistent with several recent reports, which show that the activation and overexpression of BTAK is more frequent in early stage/low-grade ovarian tumors 46 and is proved to be an early event in tumorigenesis in a rat mammary carcinogenesis model.47
Another interesting finding is that BTAK expression by immunohistochemistry was correlated with p53 expression. p53 is a well-known tumor suppressor gene that is mutated in nearly 50% of all human tumors.48 Previous studies have showed that overexpression of BTAK phosphorylates p53, leading to its degradation and decreasing p53 level, then inducing oncogenic transformation.49 Their results suggested that expression of BTAK induces tumorigenesis through degradation of p53 and BTAK. Phosphorylation of p53 is associated with BTAK-regulated cell cycle progression, cell survival, and transformation. These data indicate that p53 may be a physiological substrate of BTAK that may exert its function through phosphorylation of p53. Our finding of high p53 expression level, most likely indicative of mutant forms, has been shown to be associated with high BTAK expression. However, the underlying mechanisms of how the interaction of BRCA, p53, and BTAK regulate the initiation of ovarian tumorigenesis remain to be tested in future experiments.
In summary, we provide strong evidence that BTAK is overexpressed in the ovaries of women with BRCA mutation or strong family history of breast and ovarian cancer compared with normal ovaries from women who lack BRCA mutation. BTAK expression is associated with elevated mutant p53. As several inhibitors for BTAK are at various stage of clinical trials,50, 51 BTAK may offer a novel target for chemoprevention to decrease ovarian cancer risk and save patient from a drastic surgical procedure, prophylactic oophorectomy.
References
Ford D, Easton DF . The genetics of breast and ovarian cancer. Br J Cancer 1995;72:805–812.
Kennedy RD, D'Andrea AD . The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev 2005;19:2925–2940.
Shah N, Lepre J, Tu Y, et al. Can we identify cellular pathways implicated in cancer using gene expression data? Proc IEEE Comput Soc Bioinform Conf 2003;2:94–103.
Somasundaram K, MacLachlan TK, Burns TF, et al. BRCA1 signals ARF-dependent stabilization and coactivation of p53. Oncogene 1999;18:6605–6614.
Chai YL, Cui J, Shao N, et al. The second BRCT domain of BRCA1 proteins interacts with p53 and stimulates transcription from the p21WAF1/CIP1 promoter. Oncogene 1999;18:263–268.
MacLachlan TK, Dash BC, Dicker DT, et al. Repression of BRCA1 through a feedback loop involving p53. J Biol Chem 2000;275:31869–31875.
Gowen LC, Avrutskaya AV, Latour AM, et al. BRCA1 required for transcription-coupled repair of oxidative DNA damage. Science 1998;281:1009–1012.
Xu X, Weaver Z, Linke SP, et al. Centrosome amplification and a defective G2–M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell 1999;3:389–395.
Daniels MJ, Wang Y, Lee M, et al. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science 2004;306:876–879.
Salazar H, Godwin AK, Daly MB, et al Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J Natl Cancer Inst 1996;88:1810–1820.
Barakat RR, Federici MG, Saigo PE, et al. Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer 2000;89:383–390.
Schlosshauer PW, Cohen CJ, Penault-Llorca F, et al. Prophylactic oophorectomy: a morphologic and immunohistochemical study. Cancer 2003;98:2599–2606.
Katayama H, Brinkley WR, Sen S . The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metast Rev 2003;22:451–464.
Meraldi P, Honda R, Nigg EA . Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev 2004;14:29–36.
Stenoien DL, Sen S, Mancini MA, et al. Dynamic association of a tumor amplified kinase, Aurora-A, with the centrosome and mitotic spindle. Cell Motil Cytoskeleton 2003;55:134–146.
Marumoto T, Hirota T, Morisaki T, et al. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells 2002;7:1173–1182.
Giet R, Petretti C, Prigent C . Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol 2005;15:241–250.
Hu W, Kavanagh JJ, Deaver M, et al. Frequent overexpression of STK15/Aurora-A/BTAK and chromosomal instability in tumorigenic cell cultures derived from human ovarian cancer. Oncol Res 2005;15:49–57.
Zhou H, Kuang J, Zhong L, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet 1998;20:189–193.
Bischoff JR, Anderson L, Zhu Y, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J 1998;17:3052–3065.
Sakakura C, Hagiwara A, Yasuoka R, et al. Tumour-amplified kinase BTAK is amplified and overexpressed in gastric cancers with possible involvement in aneuploid formation. Br J Cancer 2001;84:824–831.
Ouchi M, Fujiuchi N, Sasai K, et al. BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. J Biol Chem 2004;279:19643–19648.
Hsu LC, Doan TP, White RL . Identification of a gamma-tubulin-binding domain in BRCA1. Cancer Res 2001;61:7713–7718.
Xu X, Wagner KU, Larson D, et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet 1999;22:37–43.
Rosen DG, Yang G, Bast Jr RC, et al. Use of ras-transformed human ovarian surface epithelial cells as a model for studying ovarian cancer. Methods Enzymol 2005;407:660–676.
Rosen DG, Yang G, Deavers MT, et al. Cyclin E expression is correlated with tumor progression and predicts a poor prognosis in patients with ovarian carcinoma. Cancer 2006;106:1925–1932.
Stratton JF, Buckley CH, Lowe D, et al. Comparison of prophylactic oophorectomy specimens from carriers and noncarriers of a BRCA1 or BRCA2 gene mutation. United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group. J Natl Cancer Inst 1999;91:626–628.
Casey MJ, Bewtra C, Hoehne LL, et al. Histology of prophylactically removed ovaries from BRCA1 and BRCA2 mutation carriers compared with noncarriers in hereditary breast ovarian cancer syndrome kindreds. Gynecol Oncol 2000;78:278–287.
Piek JM, Verheijen RH, Menko FH, et al. Expression of differentiation and proliferation related proteins in epithelium of prophylactically removed ovaries from women with a hereditary female adnexal cancer predisposition. Histopathology 2003;43:26–32.
Qi Cai K, Klein-Szanto A, Karthik D, et al. Age-dependent morphological alterations of human ovaries from populations with and without BRCA mutations. Gynecol Oncol 2006;103:719–728.
Colgan TJ, Murphy J, Cole DE, et al. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol 2001;25:1283–1289.
Deligdisch L, Gil J, Kerner H, et al. Ovarian dysplasia in prophylactic oophorectomy specimens: cytogenetic and morphometric correlations. Cancer 1999;86:1544–1550.
Bell DA . Origins and molecular pathology of ovarian cancer. Mod Pathol 2005;18 (Suppl 2):S19–S32.
Dyck HG, Hamilton TC, Godwin AK, et al. Autonomy of the epithelial phenotype in human ovarian surface epithelium: changes with neoplastic progression and with a family history of ovarian cancer. Int J Cancer 1996;69:429–436.
Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol 2000;18:2728–2732.
Werness BA, Afify AM, Bielat KL, et al. Altered surface and cyst epithelium of ovaries removed prophylactically from women with a family history of ovarian cancer. Hum Pathol 1999;30:151–157.
Roland IH, Yang WL, Yang DH, et al. Loss of surface and cyst epithelial basement membranes and preneoplastic morphologic changes in prophylactic oophorectomies. Cancer 2003;98:2607–2623.
Auersperg N, Wong AS, Choi KC, et al. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 2001;22:255–288.
Kruk PA, Godwin AK, Hamilton TC, et al. Telomeric instability and reduced proliferative potential in ovarian surface epithelial cells from women with a family history of ovarian cancer. Gynecol Oncol 1999;73:229–236.
He QY, Zhou Y, Wong E, et al. Proteomic analysis of a preneoplastic phenotype in ovarian surface epithelial cells derived from prophylactic oophorectomies. Gynecol Oncol 2005;98:68–76.
Hirota T, Kunitoku N, Sasayama T, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell 2003;114:585–598.
Sen S, Zhou H, White RA . A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene 1997;14:2195–2200.
Tanner MM, Grenman S, Koul A, et al. Frequent amplification of chromosomal region 20q12–q13 in ovarian cancer. Clin Cancer Res 2000;6:1833–1839.
Okada S, Ouchi T . Cell cycle differences in DNA damage-induced BRCA1 phosphorylation affect its subcellular localization. J Biol Chem 2003;278:2015–2020.
Andrews PD, Knatko E, Moore WJ, et al. Mitotic mechanics: the auroras come into view. Curr Opin Cell Biol 2003;15:672–683.
Gritsko TM, Coppola D, Paciga JE, et al. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res 2003;9:1420–1426.
Goepfert TM, Adigun YE, Zhong L, et al. Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res 2002;62:4115–4122.
Morgan SE, Kastan MB . p53 and ATM: cell cycle, cell death, and cancer. Adv Cancer Res 1997;71:1–25.
Wang X, Zhou YX, Qiao W, et al. Overexpression of aurora kinase a in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene 2006;25:7148–7158.
Hata T, Furukawa T, Sunamura M, et al. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res 2005;65:2899–2905.
Vankayalapati H, Bearss DJ, Saldanha JW, et al. Targeting aurora 2 kinase in oncogenesis: a structural bioinformatics approach to target validation and rational drug design. Mol Cancer Ther 2003;2:283–294.
Acknowledgements
JL was supported by a Research Scholar Grant (RSG-04-028-1-CCE) from American Cancer Society, Institutional Research Grant, and MD Anderson SPORE in Ovarian Cancer. The authors acknowledge Drs Susan Davidson, Gynecologic-Oncology surgeon, and Ali Akalin, Fellow in Surgical Pathology from the University of Colorado and Health Sciences Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Singh, M., Davidson, S. et al. Activation of BTAK expression in primary ovarian surface epithelial cells of prophylactic ovaries. Mod Pathol 20, 1078–1084 (2007). https://doi.org/10.1038/modpathol.3800945
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800945
Keywords
This article is cited by
-
Effect of AURKA Gene Expression Knockdown on Angiogenesis and Tumorigenesis of Human Ovarian Cancer Cell Lines
Targeted Oncology (2016)
-
AURKA and BRCA2 expression highly correlate with prognosis of endometrioid ovarian carcinoma
Modern Pathology (2011)
-
The molecular mechanism studies of chirality effect of PHA-739358 on Aurora kinase A by molecular dynamics simulation and free energy calculations
Journal of Computer-Aided Molecular Design (2011)