Abstract
The 2002 TNM formulation defines a pT3b tumor as one that ‘extends into the renal vein or its segmental (muscle containing) branches.’ This definition elicits uncertainty when veins with little muscle are involved or the relationship to the main renal vein is unknown. The diameter and medial thickness of 10 normal renal venous systems were studied and compared to sinus veins involved in 54 pT3b clear cell renal cell carcinomas (CC). All tumors were grossly examined and sampled for histology by the author. An immunoperoxidase cocktail containing CD 31 and actin, Masson trichrome and elastic stains were employed to aid identification of intravenous tumor. The venous dissections showed variable numbers of primary and secondary divisions with substantial overlap in diameter and medial thickness. The medial thickness decreased with each proximal division and ranged from being nonexistent to being thick. Study of the 54 pT3b CC revealed that the initial phase of extrarenal extension involved large caliber veins draining the primary tumor. With extensive venous involvement, tumor invaded through the vein wall into sinus fat or demonstrated retrograde venous extension into adjacent cortex. Correlation between gross and histology revealed that most nodules of tumor within the sinus fat contained evidence of pre-existing veins. The following observations were made: (1) the diameter of a sinus vein or the quantity of muscle is a poor indicator of vein segment or relationship to the main renal vein; therefore, the wording used to define pT3b should be clarified; (2) extrarenal spread in CC begins with intravenous extension whereas sinus fat invasion is usually secondary; (3) retrograde venous extension occurs in cases with massive renal vein involvement; and (4) nodules within the sinus fat usually represent venous involvement.
Similar content being viewed by others
Main
The fifth edition of the American Joint Commission of Cancer TNM staging manual published in 2002 introduced several modifications in the staging of renal cell carcinomas (CCs).1 One modification is of importance to this study; renal sinus fat and renal sinus vein invasion were included as pT3a and pT3b staging parameters, respectively. The rationale for including renal sinus involvement in pT3 is the evidence that renal CC preferentially extends beyond the kidney by invasion of the renal sinus.2, 3, 4 Recently published outcome data from the Mayo Clinic have validated this revision, with the demonstration that the prognosis for sinus invasion is very poor, with a 5-year survival of only 26%.5
Renal sinus invasion is usually associated with invasion of renal sinus veins, accounting for the predilection of clear cell renal CC to metastasize to lung, liver, bone and brain. The 2002 TNM formulation defines a pT3b tumor as one that ‘extends into the renal vein or its segmental (muscle containing) branches.’ The terminology, ‘segmental or (muscle containing) branches,’ may elicit staging uncertainty when sinus veins with little discernable muscle are involved, or when in a histologic section the relationship of an involved vein to the main renal vein is unknown.1 In this study, the gross and histologic features of 10 normal renal venous systems, and the gross and histologic features of 54 sinus vein invasive clear cell renal CCs were studied, to clarify the architecture and histologic features of renal sinus veins, and to develop criteria for gross and histologic recognition of renal sinus vein involvement.
Materials and methods
Renal Venous System Dissections
The venous systems of 10 autopsy and surgical kidneys, five right side and five left side, were dissected by careful removal of sinus fat. The main renal vein, its primary and secondary tributaries, and occasionally a tertiary division were isolated. The specimens were formalin-fixed, and 5–10 cross-sections or longitudinal sections of whole-mount preparations were histologically examined with hematoxylin and eosin (H&E) stain and Masson trichrome stain.
Clear Cell Renal CC
Fifty-four cases of renal sinus vein invasive (pT3b) CC were selected for study. Three dissection strategies were employed to assess renal sinus vein invasion.1 Fifty-two cases were opened along a probe inserted into the collecting system and poked through the lateral aspect of the tumor, our usual dissection approach.2 Ten tumors were also opened along the venous system by placing one or more probes within the renal veins, poking the probes through the lateral aspect, and then opening along the probes. This allowed direct gross visualization of tumor within sinus veins.3 In two cases with main renal vein involvement by tumor, much of the surrounding sinus fat was removed exposing intravenous tumor to gross examination. Thirty cross-sections (14 and 16 sections per case) of grossly involved veins were submitted for histologic examination.
Histologic examination in all 54 cases included a minimum of five blocks of tissue from the tumor–renal sinus interface. Histologic examination in 40 cases included two blocks of sinus vein invasive tumor immunoperoxidase stained using a cocktail containing of CD 31 and actin (Abcam, 1:1000). Masson trichrome stains and elastic stains were performed on selected cases.
Results
Normal Renal Sinus Veins
Gross features
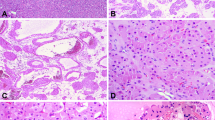
The dissected venous systems consisted of the main renal vein and its primary and secondary tributaries (Figure 1). No two dissections were alike. In several specimens, 1–10 mm lateral anastomoses that connected primary or secondary tributaries were present. In three cases, a tertiary tributary was removed intact, but this tested the limits of the author's dissection ability. The feature limiting the completeness of the dissections was the thinness of the venous wall more than the caliber of the vein. When tertiary divisions were removed, they represented intralobar veins at their exit point from the renal parenchyma.
This dissected left renal venous system consists of three primary tributaries; two have lateral anastamoses of 1 and 3 mm in diameter (arrows). The thickest primary division is a direct extension of the main renal vein. In contrast, a primary and a secondary tributary have thin transparent walls (arrowheads).
The diameters of the main renal veins ranged from 0.7 to 1.5 cm. The primary tributaries of the main renal vein ranged in number from 2 to 4 per specimen, and their diameters were comparable to those of main renal veins and ranged from 0.4 to 1.5 cm. The diameters of the secondary tributaries were only slightly smaller than the primary tributaries; they ranged from 0.2 to 1.0 cm. Grossly, the vein walls differed noticeably in thickness. The thickest segment was the portion of the main renal vein and the primary division arising as a direct linear extension of the main renal vein. The vein wall thickness decreased with each successive division. There were major differences in vein thickness within a division in a given dissection, and between similar levels of division in separate dissections. Some primary divisions were thick like the main renal vein, whereas an adjacent primary division appeared thin to the point of transparency (Figure 1).
Histologic features
Histologic sections of the venous dissections showed the typical smooth muscle fascicular arrangement of veins with fascicles separated by collagenous tissue (Figure 2). The main renal vein medial smooth muscle layer ranged from 0.35 to 0.7 mm in thickness, and the largest proximal tributaries of the main renal vein varied from 0.1 to 0.7 mm, with most measurements in the 0.2–0.4 mm range. The secondary tributaries were noticeably thinner; their smooth muscle media ranged from 0.0 to 0.4 mm (Figure 3). The 0.0 mm measurement reflects segments of discontinuous smooth muscle where only an intimal and adventitial layer comprised the vein wall. The closer a vein to its exit point from the renal parenchyma, the thinner its medial layer (Figure 4). The thickness of the smooth muscle media in a cross-section varied considerably in all venous divisions, where the media may be 2–3 × thick on one side compared to the opposite side, or compared to an adjacent segment in a longitudinal section (Figures 2, 3 and 4).
Clearly, the dissections were not complete representations of the venous systems; rather they represent a simplified shadow compared to the lush venous labyrinth revealed by venous casting (Figure 5).6 Nevertheless, these dissections provided an opportunity to appreciate the variability in architecture and thickness of sinus veins, and thereby, provide a perspective useful for gross and histologic correlation of renal tumors that involve the sinus veins.
Gross Features of Intravenous Tumor
Kidneys opened along the collecting system
Kidneys bivalved through the collecting system demonstrated the relationship between the primary tumor, and the renal pelvis and sinus. The primary tumor in some sinus vein invasive tumors was limited to the cortex, whereas in other cases, the tumors extended into the sinus fat or when large obliterated a sizable portion of the sinus impinging upon the collecting system. In many cases within the sinus fat and separate from the primary tumor, discrete circular to elongated nodules of tumor were visible (Figure 6). In tumors with extensive venous involvement, especially those that massively involved the main renal vein, nodules or cords of tumor extended toward the cortex, often bifurcating, entering the cortex in a column of Bertin between two renal pyramids, or encircling the corticomedullary junction (Figure 7). Histologic examination revealed in many cases that the nodules and cords of tumor, represented tumor within renal sinus veins or within renal cortical veins (arcuate and intralobular) as discussed below. The latter are regarded as retrograde venous involvement.
Kidneys opened along renal veins
Ten kidneys were opened along renal sinus veins permitting direct visualization of tumor and its relationship with the primary tumor. In five cases, the intravenous tumor extended to the surgical margin of the main renal vein. In the remaining five cases, the cylinder of intravenous tumor extended up to several centimeters into sinus veins and ended with a round terminus (Figure 8). The intravenous tumor was cylindrical and dilated the involved vein. Four cases had two or more longitudinal profiles of sinus veins filled with tumor. In nine of 10 cases, there was continuity between the primary tumor and intravenous tumor. In one case, a 2 mm bright yellow nodule of tumor was observed adherent to the vein lumen within the sinus, discontinuous relative to the main tumor (Figure 9). This patient had a previously documented pulmonary metastasis. Had this specimen not been opened through the renal veins, it is unlikely that venous involvement would have been identified and the patient would have been under-staged and given a pT1b designation.
This primary tumor is separated from the sinus by a layer of uninvolved cortex. Tumor can be seen extending into the dilated vein from within the cortical primary. There is a separate tiny nodule of tumor within a sinus vein (arrow). This patient had a biopsy-proven pulmonary metastasis. Tumor (IVT) can be seen extending into the dilated vein (V) from the cortical primary.
Histologic Features of Intravenous Tumor
The bivalved kidneys
Intravenous tumor extensions within renal sinus veins were usually easily identified by the presence of a smooth muscle media completely, or partially, enveloping the tumor nodules (Figure 10a and b). In most cases, the H&E stain was sufficient to recognize the presence of intravenous involvement. This interpretation was facilitated by having a minimum of five blocks available for review as not every rounded or elongated profile of tumor within the sinus had a recognizable investing smooth muscle media. The intravenous tumor may attach to the vein wall or be separated by luminal space. When there is separation between tumor and the media, or when a terminal extension (leading front) of tumor within a vein was encountered, the tumor was usually covered by a layer of CD31-positive endothelium. In a single vein in two cases, an endothelial cell covering was absent at an intravenous extension. Fibrin and blood clot were associated with disrupted tumor, indicating an in vivo event (Figure 10b).
Intravenous extensions consistently demonstrated two distinctive features. They had an extensive delicate capillary lattice, identical to that observed in the primary tumors, and the intravenous capillary lattice appeared supplied by multiple vascular pedicles that sprouted from the vein wall along the involved vein segments (Figures 10, 11 and 12). The vascular pedicles were characterized by small veins oriented perpendicular to the media that extended a short distance into the intravenous tumor, and branched to supply the intravenous capillary plexus (Figure 11). Within the largest involved veins, well-formed veins with a prominent smooth muscle media were observed within the central portions of intravenous tumor (Figure 12). Elastic stains failed to demonstrate an arterial structure in the vascular pedicles as defined by the presence of a distinct internal elastic lamina. When tumor involved the smallest caliber venous structures, venules to capillary-sized structures, both vascular pedicles and a capillary plexus were absent. Some of these small, involved spaces lacking a capillary plexis may represent lymphatic involvement as reported previously, easily addressed with an immunohistochemical stain for a lymphatic endothelial cell marker.7
Not all discrete nodules of tumor within the sinus were recognizable as intravenous tumor on H&E-stained sections. Some required immunohistochemistry (IH) for confirmation. When no smooth muscle media was apparent, however, the corresponding CD31-SMA stain frequently confirmed features of a pre-existing vein. Overall, three groups that differed in extent of venous involvement were identified. Four tumors had a single vein involved without invasion of the media or extension into the sinus fat. The primary tumors were 2.0, 3.8, 4.5 and 6.5 cm in diameter. There were 32 cases with either multiple involved veins without sinus fat involvement, or multiple-involved veins with limited extension of tumor through the media into the sinus fat leaving almost all of the media intact (Figures 13, 14 and 15). These cases did not require IH for confirmation of venous involvement. There were 18 cases with both intravenous tumor and extensive sinus fat involvement. In many nodules of tumor within sinus fat, IH revealed remnants of a centrally located vein regarded as representing tumor that had circumferentially invaded through the media of veins, leaving partial or complete silhouettes of the media (Figures 16 and 17).
(a) The presence of underlying veins is difficult to appreciate in this multinodular mass of tumor within sinus fat. (b) Actin stain of the multinodular mass of tumor in a reveals a small vein (V) at the top and substantial remaining portions of the media (arrow) of a larger vein below. (b) immunoperoxidase CD31/smooth muscle actin.
Finally, there were some nodules in which IH did not reveal medial smooth muscle fascicles, precluding confirmation of an involved vein. By definition, involved veins were noted elsewhere qualifying for the pT3b designation. Although unproven, when these nodules were separate from the primary tumor, they were regarded as intravenous tumor with complete circumferential media destruction and stromal extension. Other nodules of tumor, often adjacent to the primary tumor, were within a mesh of poorly defined smooth muscle. IH revealed sheets of individual actin-positive cells without fascicular structure. These were regarded as tumor within a desmoplastic stroma.
Dissections of grossly involved sinus veins
In two cases with massive main renal involvement by tumor, probes were inserted into grossly involved sinus veins that contained contiguous cylinders of tumor extending from the primary tumor to the margin of the main renal vein (Figure 18a). The majority of the sinus fat was dissected, and 30 cross-sections of grossly validated intravenous tumor were examined histologically. In 23 of 30 cross-sections, there was little or no residual evidence of venous media by H&E and Masson trichrome stain (Figure 18b). Tumor had circumferentially extended into the perivenous sinus fat obliterating all evidence of the renal vein structure.
Discussion
The AJCC 2002 TNM staging system defines pT3b as a tumor that extends into the main renal vein or ‘its segmental (muscle containing) branches.’ The wording, ‘segmental or (muscle containing) branches,’ lacks precision and anatomic specificity.1 This can lead to uncertainty when sinus veins with little discernable muscle are involved, or when in a histologic section, the size or relationship of an involved vein to the main renal vein is unknown. These uncertainties prompted this study of the architecture and histology of normal renal sinus veins, and detailed investigation of venous involvement in 54 pT3b clear cell renal CCs to clarify criteria for the gross and histologic recognition of venous involvement.
Normal Renal Venous System
There have been numerous studies of the renal venous system and all have revealed an extraordinarily profuse venous labyrinth that drains each kidney.8, 9, 10 This venous profusion results from the abundant arterial supply to the kidneys. Each receives 10% of the cardiac output with only a fraction converted to urine; the remaining returns via the venous outflow.
Although there are countless variations in the architecture of the renal veins, there are a few generalizations useful to keep in mind when examining a nephrectomy specimen. The left and right main renal venous systems differ. The left renal vein is longer than the right as it passes over the aorta to reach the vena cava. The left renal vein is often single, whereas the right renal vein will often have two or three major connections to the vena cava. Finally, the left renal vein has a more complicated system of extrarenal tributaries that include the adrenal, gonadal, and posterior lumbar veins that join the renal vein after it exits the renal sinus. On the right side, these veins usually join directly to the vena cava. These venous connections facilitate tumor spread to the axial skeleton and pelvis, and other sites. Within the renal sinus, tributaries of the main renal vein receive drainage from intralobar veins that exit the anterior and posterior renal parenchyma. Multiple2, 3 venous divisions converge anterior to the renal pelvis to eventually form the main renal vein(s). These tributaries are connected by numerous variably sized anastomoses forming a venous labyrinth within the renal sinus.
The architectural diversity of renal veins was apparent in the 10 dissected venous systems of this study, as no two systems were the same. Although the dissections varied in number of primary and secondary divisions, each appeared to have 2–3 generations of tributaries between the renal parenchyma and the main renal vein. There was substantial overlap in diameter between the main renal vein and its primary and secondary tributaries, and multiple lateral anastomoses were noted. The variability in gross architecture was paralleled by variability in the measured thickness of media smooth muscle of each division. Although the medial thickness progressively decreased with each proximal division of veins, the media alternated between very thin to nonexistent, to much thicker by two- to threefold. However, in contrast to cortical veins that lack a smooth muscle media, all sinus veins had medial smooth muscle, albeit at times, thin or interrupted.
These observations have practical implications when reviewing histologic sections showing sinus vein involvement by tumor. All primary, secondary, and tertiary tributaries of the main renal vein are sizable veins. All are within 1–2 cm to the main renal vein and may have a generous or scant smooth muscle media. Furthermore, a millimeter-sized vein may represent an anastomoses between large renal vein tributaries, and if involved, would indicate involvement of a large caliber vein. Therefore, the diameter of the vein involved or the quantity of smooth muscle in a histologic section, is a poor indicator of the vein segment or its relationship to the main renal vein.
Renal Sinus Involvement in Clear Cell Renal CC
Correlation between the gross and microscopic features of sinus involvement demonstrated that in most instances a round to elongate tumor nodule within the sinus fat contained evidence of an involved vein. In most instances, this was easily demonstrated by routine H&E-stained sections, in at least some of the nodules. However, other nodules required an actin stain to unveil an underlying, usually centrally located vein.
The microscopic features of intravenous tumor had several features of interest. The intravenous tumor is clearly not a tumor thrombus, as commonly referenced, but rather is an actively growing extension from the primary tumor. The intravenous tumor typically had a delicate supporting capillary lattice, identical to the primary tumor, and characteristic of CC in general. The presence of a capillary lattice is diagnostically useful, as recently shown by Bonsib,7 because it distinguishes a vein from a lymphatic, a distinction also possible with IH. This capillary lattice is nourished by numerous venous pedicles that sprout from the media at numerous foci along the intravenous extension. The cylinder of intravenous tumor usually had an endothelial cell investment when not adherent to the vein wall. This feature coupled with the supporting capillary plexus may provide some degree of structural integrity, and possibly resistance to fragmentation and tumor embolization.
Tumors vary greatly in the extent of sinus involvement. When cases with limited venous involvement were compared with cases with limited sinus fat and widespread sinus fat involvement, the sequential phases of sinus involvement could be inferred permitting creation of a typical paradigm for CC extending beyond the kidney. The invasive process characteristically begins with tumor directly entering large caliber sinus veins while the tumor is otherwise limited to the parenchyma. With more extensive venous involvement, tumor may dissect through the vein wall and extend into the sinus fat. However, some cases with massive venous involvement extending to the main renal vein may show no detectable involvement of sinus fat.
Direct extension of tumor into sinus fat from the primary tumor appears infrequent compared to the transvenous route, and did not represent the initial phase of sinus involvement in the 54 cases of this study. Evidence for the primacy of sinus venous involvement was clearly provided in 36 of 54 cases, where there was unequivocal evidence that sinus fat involvement arose solely by invasion through the wall of sinus veins rather than by direct invasion from the primary. In 18 of 54 cases that had extensive venous and sinus fat involvement, this relationship was more difficult to establish. However, even in these cases, most tumor nodules contained a centrally located portion of a vein demonstrable by actin staining, suggesting that sinus fat invasion derived from that vein. As in this study large veins appeared to be involved first whereas sinus fat involvement appeared to follow intravenous growth, involvement of any size sinus vein or the presence of tumor in sinus fat suggests that large veins are involved somewhere. The ability of CC to completely obliterate all evidence of a pre-existing vein, however, means that a residual vein may not always be identified in a given section. However, vein obliteration was only observed in cases with extensive venous involvement. Additional sections of tumor within the sinus or the use of an actin stain should allow correct stage to be assigned. Routine use of the transvenous dissection approach may minimize this problem as discussed next.
The use of a transvenous dissection approach was very informative. It validated the primacy of venous involvement in cases where tumor was grossly observed extending into large sinus veins whereas the primary was limited to the cortex. Intravenous dissection also led to two additional important observations. In a number of cases, tumor nodules, millimeter to centimeter in size, were observed in the cortex distant from the primary tumor, in a pattern or in a location consistent with renal parenchymal veins. That is, cords of tumor could be observed entering between renal pyramids and arrayed along the corticomedullary junction where intralobar and arcuate veins travel. Furthermore, some nodules would bifurcate toward the cortex, consistent with the confluence of two veins heading toward the sinus. This phenomenon is regarded as retrograde venous involvement. Corresponding histology often supported this interpretation of venous involvement distant from the primary tumor. However, as cortical veins lack a smooth muscle media, only small nodules could be convincingly demonstrated to represent veins by virtue of their juxta-arterial location and/or the persistence of residual endothelial lining.
Retrograde venous extension is a complication of massive main renal vein involvement. One could envision that the proximal tributaries draining portions of the kidney not involved by tumor may represent favored avenues for continued tumor growth when the main renal vein is occluded by tumor. The existence of retrograde venous extension with growth of separate cortical nodules may be relevant to the issue of multifocality of renal CCs. The literature contends that as many as 5% of renal CCs may be multifocal.11, 12 However, the concept of retrograde venous extension in renal CC may not have been considered in these studies. As cortical veins lack a smooth muscle media, it is difficult on histologic grounds to investigate this possibility except for microscopic foci. Although only one of 54 cases studied herein were regarded as having a second primary tumor, this series is too small to adequately address the issue of retrograde extension vs a second primary tumor.
In addition to appreciation of retrograde venous extension, the transvenous dissection approach permitted the identification of a pT3b tumor that would otherwise have been designated pT1b. This was the case with a discontinuous nidus of tumor within a sinus vein (Figure 9) in which the patient had a histologically confirmed pulmonary metastasis. Had this specimen been opened through the collecting system or by another method, it is likely that this minimal evidence of extrarenal extension would have been missed. This case provides a graphic illustration of how a venous metastasis may occur while the primary tumor may otherwise appear renal-limited. It also indicates that no matter how thoroughly a tumor is examined, or what refinements of T staging are implemented, we will never be able to forecast 100% survival based upon T staging of the primary tumor.
Conclusions
The diameter of a sinus vein or its quantity of smooth muscle, is a poor indicator of the vein segment involved or its relationship to the main renal vein. Therefore, the wording used to define pT3b in the AJCC 2002 TNM staging system should be clarified and linked to some feature demonstrable histologically, possibly the presence of a capillary lattice. This study of venous involvement in CC demonstrated that intravenous extension is the first step in extrarenal spread. With progression, there is secondary sinus fat invasion, and in tumors with extensive sinus vein involvement, retrograde venous growth can involve other regions of the kidney. Most nodules within the sinus represent venous involvement. However, histologic confirmation can be difficult when tumor has obliterated the pre-existing vein. Submission of additional blocks of tumor or staining for smooth muscle actin to reveal residual venous media will usually resolve uncertainty. Use of transvenous dissection provides the best opportunity to recognize venous extension in the gross room and sample accordingly, and may reduce the need for additional blocks and IH.
References
Green FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual, 6th edn. Springer: New York, 2002.
Bonsib SM . The renal sinus is the principal invasive pathway: a prospective study of 100 renal cell carcinomas. Am J Surg Pathol 2004;28:1594–1600.
Bonsib SM . T2 clear cell renal cell carcinoma is a rare entity: a study of 120 clear cell renal cell carcinomas. J Urol 2005;174:1199–1202; discussion 1202.
Bonsib SM, Gibson D, Mhoon M, et al. Renal sinus involvement in renal cell carcinomas. Am J Surg Pathol 2000;24:451–458.
Thompson RH, Leibovich BC, Cheville JC, et al. Is renal sinus fat invasion the same as perinephric fat invasion for pT3a renal cell carcinoma? J Urol 2005;174:1218–1221.
Satyapal KS . Classification of the drainage patterns of the renal veins. J Anat 1995;186 (Part 2):329–333.
Bonsib SM . Renal lymphatics, and lymphatic involvement in sinus vein invasive (pT3b) clear cell renal cell carcinoma: a study of 40 cases. Mod Pathol 2006;19:746–753.
Anson BJ, Cauldwell EW, Pick JW, et al. The anatomy of the pararenal system of veins, with comments on the renal arteries. J Urol 1948;60:714–737.
Gillot C . The left renal vein. Anat Clin 1978;1:135–156.
Pick JW, Anson BJ . The vascular pedicle, an anatomic study of 430 body-halves. J Urol 1940;44:411–429.
Richstone L, Scherr DS, Reuter VR, et al. Multifocal renal cortical tumors: frequency, associated clinicopathological features and impact on survival. J Urol 2004;171:615–620.
Ritchey ML, Shamberger RC, Hamilton T, et al. Fate of bilateral renal lesions missed on preoperative imaging: a report from the National Wilms Tumor Study Group. J Urol 2005;174:1519–1521; discussion 1521.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonsib, S. Renal veins and venous extension in clear cell renal cell carcinoma. Mod Pathol 20, 44–53 (2007). https://doi.org/10.1038/modpathol.3800726
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800726
Keywords
This article is cited by
-
Retrograde venous invasion in renal cell carcinoma: a gross diagnosis
Surgical and Experimental Pathology (2023)
-
WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: standards and controversies
World Journal of Urology (2018)
-
WHO-Klassifikation von 2016 und erste S3-Leitlinie zum Nierenzellkarzinom
Der Pathologe (2016)
-
Biomarker für Nierenkarzinome
Der Pathologe (2012)