Abstract
Tubulolobular carcinoma is a type of mammary carcinoma that displays an admixture of invasive tubules and lobular-like cells. Previous reports have shown it to share clinical similarities to lobular carcinoma, whereas more recent studies have shown it to be E-cadherin positive. The aim of the current study was to further explore the immunophenotype of tubulolobular carcinoma, and to document its natural behavior. Nineteen cases of tubulolobular carcinoma and 10 cases each of tubular and lobular carcinoma were retrieved for comparison analysis. Immunohistochemistry was performed with antibodies against estrogen receptor, progesterone receptor, HER2/neu, 34βE12, E-cadherin, and the catenins. Twenty-five percent of patients with tubulolobular carcinoma presented with greater than stage I disease, compared to 0 and 60% of patients with tubular and lobular carcinoma, respectively. Two patients with tubulolobular carcinoma had tumor recurrence, one of whom also developed metastasis. The majority of all carcinomas were estrogen and progesterone receptor positive. E-cadherin displayed membranous staining in all tubular and tubulolobular carcinomas, and was negative in all lobular carcinomas. Half of each carcinoma subtype displayed granular cytoplasmic 34βE12 immunoreactivity. α-Catenin exhibited partial or complete membranous staining in all tubulolobular and tubular carcinomas, and was negative in all lobular carcinomas. β-Catenin displayed membranous staining in tubulolobular and tubular carcinomas, whereas all lobular carcinomas had coarse cytoplasmic immunoreactivity. p120 and γ-catenin displayed membranous staining in 100% of tubulolobular and tubular carcinomas and cytoplasmic staining in 100% of lobular carcinomas. Tubulolobular carcinoma of the breast is thus a distinct type of mammary carcinoma that displays both tubular and lobular patterns histologically but displays the membranous E-cadherin/catenin complex characteristic of the ductal immunophenotype. Tubulolobular carcinoma appears to be more aggressive than tubular carcinoma, as 16% of patients had lymph node metastases, although all were alive at a mean follow-up of 40 months.
Similar content being viewed by others
Main
The distinction between ductal and lobular carcinomas of the breast can usually be made by applying typical cytologic and architectural criteria, such as tubule formation and pleomorphism in the former, and dyscohesion of tumor cells in the latter. However, some carcinomas display overlapping features, making this distinction difficult. Tubulolobular carcinoma is a distinct type of mammary carcinoma that, as its name suggests, displays an admixture of minimally pleomorphic invasive tubules, as seen in classic tubular carcinoma, and dyscohesive cells with low-nuclear grade, as seen in classic lobular carcinoma. As tubulolobular carcinomas are rare neoplasms, representing less than 3% of all breast cancers, relatively few studies aimed at their analysis have been performed, and thus little is known about their behavior. A study published in 1977 by Fisher et al1 concluded that these neoplasms, while sharing features common to both pure tubular and lobular carcinomas, are better characterized as a tubular variant of lobular carcinoma. Green et al2 found that multifocality and positive axillary lymph nodes were more frequent in tubulolobular carcinoma than in tubular carcinoma, suggesting that the former is a higher-grade lesion than tubular carcinoma and shares more clinical similarities to lobular carcinoma. Recently, Wheeler et al3 reported E-cadherin and 34βE12 positivity in tubulolobular carcinomas, suggesting that they exhibit a ‘hybrid’ ductal and lobular immunophenotype.
E-cadherin, an invasion suppressor gene, codes for a transmembrane glycoprotein that functions in intercellular adhesion.4 The E-cadherin protein internal domain binds with alpha, beta, gamma, and p120 catenins to anchor the E-cadherin complex to the actin cytoskeleton of the cell. E-cadherin is often mutated in lobular neoplasia, resulting in the absence of the extracellular E-cadherin domain and lack of membrane immunostaining for the protein.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 In contrast, mammary carcinomas that are ductal in cell origin almost universally display distinct membrane staining for E-cadherin, although high-grade ductal carcinomas show diminished E-cadherin or rarely, may be E-cadherin negative.16 Thus, immunohistochemistry for the E-cadherin protein has become a valuable diagnostic tool to differentiate ductal from lobular carcinomas.
The aim of this study was twofold: to examine the natural behavior of tubulolobular carcinomas and to immunohistologically document the phenotype as ductal or lobular, with special reference to the structure of the E-cadherin/catenin complex.
Materials and methods
Nineteen cases of tubulolobular carcinoma were selected for this IRB-approved study from the archives of Magee-Womens Hospital of UPMC and UPMC Shadyside, Department of Pathology, after reviewing all cases from a 7-year period (1997–2004) with a diagnosis of ‘tubulolobular carcinoma’, or ‘carcinoma with both ductal and lobular features’. Cases were included only if they displayed the histologic criteria put forth by Fisher et al1 and consisted of resection specimens displaying tubulolobular carcinoma morphology only or core needle biopsy specimens in which subsequent resection specimens were performed. Additionally, 10 cases each of classic tubular carcinoma and lobular carcinoma were retrieved for comparison analysis.
Thorough review of patient medical charts was conducted to obtain the following information: (1) age and stage at diagnosis, (2) laterality, (3) use of adjuvant treatment, (4) type of surgery, (5) patient history of breast cancer, and (6) time interval and status at follow-up. Pathologic characteristics were obtained by reviewing hematoxylin and eosin-stained slides and recording: (1) Nottingham scores, (2) lymphovascular invasion, (3) tumor-associated microcalcifications, (4) type of in situ component, if present, (5) association with atypical epithelial hyperplasia, and (6) multifocality.
The antibodies, clones, dilutions, pretreatment conditions, and sources for immunohistochemical studies are listed in Table 1. Estrogen receptor (ER), progesterone receptor (PR), and HER2/neu studies initially performed at the time of diagnosis were reviewed. When unavailable, formalin-fixed, paraffin embedded tissue blocks were cut at 4 μm and immunoassayed with the antibodies listed. The Envision Plus (Dako, Carpinteria, CA, USA) detection system was used for antibodies against ER, PR, HER2/neu, and E-cadherin. iVIEW DAB (Ventana, Tucson, AZ, USA) detection system was used for antibodies directed against the catenins. ER and PR were considered positive if nuclear staining was present. HER2/neu was scored on a 0 to 3+ scale using standard criteria. The type and distribution of immunostaining for 34βE12, E-cadherin and the catenins were recorded and compared to normal ductal breast epithelium present on the same slide, and scored according to the following scale: 0=negative, 1+=1–25% of cells positive, 2+=26–75% cells positive, 3+=>75% cells positive.
Results
Clinical and Histopathologic Characteristics
Patient characteristics are shown in Table 2. Briefly, the average age of patients with tubulolobular carcinoma, tubular carcinoma, and lobular carcinoma was 61, 58, and 64 years old, respectively. Two patients with tubulolobular carcinoma had a history of invasive ductal carcinoma 6 years (case no. 14, ipsilateral breast) and 2 years (case no. 18, contralateral breast) prior. None of the patients with tubular carcinoma had a personal history of breast cancer, whereas two of the patients with lobular carcinoma had a history of invasive ductal carcinoma (case nos. 31 and 35, both contralateral breasts). The majority (66%) of patients with tubulolobular carcinoma underwent segmental mastectomy, whereas 17% each were treated with modified radical mastectomy or total mastectomy. Surgical history was unknown in one case. Follow-up time ranged from 11 to 101 months (mean=40.7 months), and was unavailable in one case. Of patients with lobular carcinoma, 60 and 30% underwent segmental mastectomy or either modified radical mastectomy or total mastectomy, respectively. One patient (case no. 30) with bilateral disease was treated with right total mastectomy and left modified radical mastectomy. A summary of stage at presentation for those patients who were completely staged at time of initial diagnosis is shown in Table 3. Histopathologic characteristics are summarized in Table 4.
Figure 1 shows the typical histologic features seen in tubulolobular carcinomas (Figure 1a–d), tubular carcinomas (Figure 1e), and lobular carcinomas (Figure 1f). Tubulolobular carcinomas were characterized by an admixture of tiny round invasive tubules with minimal nuclear pleomorphism and single cells infiltrating in single-files and in targetoid fashion around non-neoplastic ducts. These tubules lacked the apical snouts and the angulated or comma shapes of tubular carcinoma. Three cases (case no. 5, 21, and 25) were associated with perineural invasion, and lymphovascular invasion was seen in only one (case no. 25). Three patients presented with lymph node metastases (3/16 completely staged cases or 19%), two of which were available for histologic review. Both lymph node metastases showed predominantly tubules, one with cribriforming glands with variably sized lumens. Both demonstrated cells with higher-nuclear grade than the primary tumor and with more abundant eosinophilic cytoplasm (Figure 2). Two additional patients (case no. 14 and 25) had tumor recurrence approximately 5 and 6 years after their original diagnosis, respectively. Both recurrent tumors, similar to the lymph node metastases, were composed predominantly of tubules and characterized by cells with apocrine features, including nuclei with prominent nucleoli and eosinophilic cytoplasm. There were some single cells and solid areas seen as well. The latter patient (case no. 25) was also found to have extensive osseous metastasis involving the skull, clavicle, ribs, sternum, vertebrae, pelvis, humerus, and femur by bone scan 78 weeks after her initial presentation.
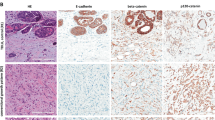
Histologic characteristics of tumors. Tubulolobular carcinomas (a–d) displayed small, round tubules infiltrating a fibrous stroma with none to mild desmoplasia, with intermixed lobular-like single cells infiltrating around ducts. Tubular carcinoma (e) showed classic well-differentiated tubules with apical snouts. Lobular carcinoma (f) exhibited low-grade single cells infiltrating around ducts in a fibrous stroma (hematoxylin and eosin).
Immunohistochemistry
Results of immunohistochemical studies are shown in Table 5, and are summarized in Table 6. The majority of all carcinomas were ER positive (95% of tubulolobular carcinomas, 100% of tubular carcinomas, 100% of lobular carcinomas) and PR positive (79% of tubulolobular carcinomas, 90% of tubular carcinomas, 90% of lobular carcinomas). HER2/neu was negative (score 0 or 1+) in the majority of all tumors.
E-cadherin displayed moderate or strong complete membranous staining in all tubular and tubulolobular carcinomas, and was completely negative in all lobular carcinomas (Figure 3). 34βE12 showed granular cytoplasmic staining of variable intensity in 59% of tubulolobular carcinomas, 50% of tubular carcinomas, and 50% of lobular carcinomas, including two cases with paranuclear dot-like positivity (Figure 4). In cases in which selected slides also had an in situ component, 34βE12 demonstrated weak granular staining in the cytoplasm of DCIS cells in one of four cases, and showed patchy weak positivity in one of two cases of LCIS. Additionally, it stained the cytoplasm of myoepithelial cells as well as luminal non-neoplastic ductal epithelium.
α-Catenin showed partial or complete membranous staining in 14/14 (100%) tubulolobular carcinomas, 9/9 (100%) tubular carcinomas, and 0/10 (0%) lobular carcinomas (Figure 5). β-Catenin, similar to α-catenin, displayed membranous staining in tubulolobular and tubular carcinomas, whereas all lobular carcinomas had no cell membrane immunostaining (Figure 6). Two cases of tubular carcinoma displayed coarse cytoplasmic β-catenin positivity in addition to membranous staining. P120 displayed crisp linear membranous staining in 100% of tubulolobular and tubular carcinomas, without cytoplasmic staining, while 100% of lobular carcinomas exhibited cytoplasmic staining without appreciable membrane staining (Figure 7).
Discussion
The terminal ductal lobular unit gives rise to the two main types of mammary malignancies, ductal and lobular carcinomas. While both are associated with loss of heterozygosity (LOH) at 16q, the E-cadherin gene locus consistently shows mutations in lobular carcinoma. This molecular event has become a useful diagnostic tool to differentiate lobular from ductal neoplasia, since E-cadherin mutations result in the absence of the extracellular E-cadherin domain and resultant lack of membrane immunostaining for the protein.
Despite the molecular and histologic distinctions that have been used to define ductal and lobular carcinomas, it is not rare to encounter ‘hybrid’ carcinomas that display histologic features common to both infiltrating ductal and lobular carcinomas. Although a significant proportion of these ‘hybrid’ tumors are high-grade, tubulolobular carcinoma represents a unique tumor of low-histologic grade displaying a mixture of small round tubules and dyscohesive lobular-like cells. The results of our study, as in previous reports, demonstrate that this hybrid histologic pattern parallels its behavior: while 60% of patients with lobular carcinoma vs 0% of patients with tubular carcinoma presented with greater than stage I disease, 23% of patients with tubulolobular carcinoma presented with stage II disease, or greater. Additionally, 17% of tubulolobular carcinoma patients completely staged had axillary lymph node metastasis, compared to 0 and 20% of tubular and lobular carcinoma cases, respectively. Finally, two additional patients with tubulolobular carcinoma had tumor recurrence approximately 5 and 6 years after their original diagnosis, respectively, one of whom also exhibited extensive bony metastases. Of note, both of these latter two patients were incompletely staged at the time of original diagnosis.
While tubulolobular carcinomas appear to overlap the histologic and behavioral characteristics of tubular and lobular carcinomas, our data show that it displays a ductal immunophenotype. E-cadherin displayed membranous immunostaining in 100% of tubulolobular carcinomas and 100% of tubular carcinomas, and was negative in all lobular carcinomas. 34βE12 was positive in about half of all the carcinomas, with no significant difference seen among tumor subgroups. A previous study reported high molecular weight keratin 34βE12, which is reactive against cytokeratins 1, 5, 10, and 14, to be a useful marker in differentiating DCIS from LCIS.17 However, another study has shown it to not be a specific marker for lobular differentiation.18 Our data supports the latter conclusion, although few cases of each were included in the present study. E-cadherin thus appears to be the only currently reliable marker to differentiate ductal from lobular cancers.
E-cadherin, a transmembrane glycoprotein expressed mainly in epithelial cells, mediates calcium-dependent cell–cell adhesion.19 It interacts with the catenins (α-, β-, γ-, and p120) via one of two intracellular domains—the juxtamembrane domain and the catenin-binding domain. Either β- or γ-catenin binds to the catenin-binding domain, while the juxtamembrane domain provides ligand-binding sites for p120. The E-cadherin/catenin complex is then connected to the actin-based cytoskeleton via α-catenin.20, 21 The catenins, like E-cadherin, may be visualized immunohistochemically and normally display membranous staining in epithelial cells. Loss of E-cadherin and abnormal cytoplasmic localization of the β-, γ-, and p120 catenins characterizes lobular cells, while α-catenin is usually absent.22 In our study, the pattern of catenin expression exhibited by tubulolobular carcinomas provides further evidence that it bears an integral E-cadherin protein.
E-cadherin alterations have been correlated with the typical dyscohesive pattern seen in lobular carcinomas.16, 23 Ductal carcinomas rarely display specific E-cadherin mutations, and even in these cases E-cadherin membrane immunoreactivity is unaltered.24 We have shown that tubulolobular carcinomas display diffuse, membranous immunoreactivity for E-cadherin and the catenins, indicative of an intact E-cadherin/catenin complex. The dyscohesive pattern seen in tubulolobular carcinomas, as well as some high-grade ductal carcinomas, may be due to other alterations leading to disrupted epithelial cell junctions, and, perhaps, a resultant increase in invasive and/or metastatic capacity. Loss of catenin function via phosphorylation of tyrosine residues may account for a disruption in cell adhesion in the setting of unaltered cadherin,25 although this typically results in disintegration of the entire complex.21, 26 An alternative mechanism for loss of cell-to-cell adhesion, other than disruption of the cadherin/catenin complex in adherens junctions, is dysregulation of epithelial cell tight junctions. For example, loss of claudin-7, a member of the claudin family of tight junction proteins, was shown to be decreased in high-grade ductal lesions compared to low-grade lesions and non-neoplastic mammary epithelium,27 implicating its role in cell dyscohesion and invasion. Exploration of this family of proteins in tubulolobular carcinoma, in addition to other invasion-related factors such as matrix metalloproteinases, may thus be of interest, as the loss of cohesion as seen in tubulolobular carcinoma may account for the relative increase in associated metastases compared to tubular carcinoma.
The p120 catenin immunostaining pattern also paralleled the results of E-cadherin immunostaining in this study. P120 catenin, as part of the juxtamembranous portion of the E-cadherin/catenin complex, is normally present in the cell membrane when E-cadherin is present, indicative of the ductal phenotype, whereas it is dispersed throughout the cytoplasm when E-cadherin is absent, as in lobular neoplasia.22, 28, 29
In conclusion, tubulolobular carcinoma of the breast is a distinct type of mammary carcinoma that displays both tubular and lobular patterns histologically but displays the membranous E-cadherin/catenin complex characteristic of the ductal immunophenotype. It may thus be better termed ‘ductal carcinoma, tubulolobular subtype’, or ‘ductal carcinoma with a tubulolobular pattern’. Even in this small group of patients, tubulolobular carcinoma appears to be more aggressive than tubular carcinoma, as 16% of patients had lymph node metastases, although it carries an overall good prognosis, as all patients were alive at a mean follow-up time of 40 months.
References
Fisher ER, Gregorio RM, Redmond C, et al. Tubulolobular invasive breast cancer: a variant of lobular invasive cancer. Hum Pathol 1977;8:679–683.
Green I, McCormick B, Cranor M, et al. A comparative study of pure tubular and tubulolobular carcinoma of the breast. Am J Surg Pathol 1997;21:653–657.
Wheeler DT, Tai LH, Bratthauer GL, et al. Tubulolobular carcinoma of the breast: an analysis of 27 cases of a tumor with a hybrid morphology and immunoprofile. Am J Surg Pathol 2004;28:1587–1593.
Ásgeirsson KS, Jónasson JG, Tryggvadóttir L, et al. Altered expression of E-cadherin in breast cancer: patterns, mechanisms and clinical significance. Eur J Cancer 2000;36:1098–1106.
Kanai Y, Oda T, Tsuda H, et al. Point mutation of the E-cadherin gene in invasive lobular carcinoma of the breast. Jpn J Cancer Res 1994;85:1035–1039.
Berx G, Becker KF, Hofler H, et al. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat 1998;12:226–237.
De Leeuw WJ, Berx G, Vos CB, et al. Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol 1997;183:404–411.
Nishizaki T, Chew K, Chu L, et al. Genetic alterations in lobular breast cancer by comparative genomic hybridization. Int J Cancer 1997;74:513–517.
Berx G, Cleton-Jansen AM, Strumane K, et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 1996;13:1919–1925.
Huiping C, Sigurgeirsdottir JR, Jonasson JG, et al. Chromosome alterations and E-cadherin gene mutations in human lobular breast cancer. Br J Cancer 1999;81:1103–1110.
Vallorosi CJ, Day KC, Zhao X, et al. Truncation of the beta-catenin binding domain of E-cadherin precedes epithelial apoptosis during prostate and mammary involution. J Biol Chem 2000;275:3328–3334.
Esteller M . Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. Eur J Cancer 2000;36:2294–2300.
Droufakou S, Deshmane V, Roylance R, et al. Multiple ways of silencing E-cadherin gene expression in lobular carcinoma of the breast. Int J Cancer 2001;92:404–408.
Cheng CW, Wu PE, Yu JC, et al. Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene 2001;20:3814–3823.
Lei H, Sjoberg-Margolin S, Salahshor S, et al. CDH1 mutations are present in both ductal and lobular breast cancer, but promoter allelic variants show no detectable breast cancer risk. Int J Cancer 2002;98:199–204.
Gamallo C, Palacios J, Suarez A, et al. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol 1993;142:987–993.
Bratthauer GL, Moinfar F, Stamatakos MD, et al. Combined E-cadherin and high molecular weight cytokeratin immunoprofile differentiates lobular, ductal, and hybrid mammary intraepithelial neoplasias. Hum Pathol 2002;33:620–627.
Lacroix-Triki M, Mery E, Voigt JJ, et al. Value of cytokeratin 5/6 immunostaining using D5/16 B4 antibody in the spectrum of proliferative intraepithelial lesions of the breast. A comparative study with 34bE12 antibody. Virchows Arch 2003;442:548–554.
Takeichi M . Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991;251:1451–1455.
Nagafuchi A . Molecular architecture of adherens junctions. Curr Opin Cell Biol 2001;13:600–603.
Wang Y, Jin G, Miao H, et al. Integrins regulate VE-cadherin and catenins: Dependence of this regulation on Src, but not on Ras. Proc Natl Acad Sci USA 2006;103:1774–1779.
Sarrio D, Perez-Mies B, Hardisson D, et al. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene 2004;23:3272–3283.
Moll R, Mitze M, Frixen UH, et al. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol 1993;143:1731–1742.
Cleton-Jansen AM . E-cadherin and loss of heterozygosity at chromosome 16 in breast carcinogenesis: different genetic pathways in ductal and lobular breast cancer? Breast Cancer Res 2002;4:5–8.
Acs G, Lawton TJ, Rebbeck TR, et al. Differential expression of E-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol 2001;115:85–98.
Takeichi M . Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol 1993;5:806–811.
Kominsky SL, Argani P, Korz D, et al. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 2003;22:2021–2033.
Shibata T, Kokubu A, Sekine S, et al. Cytoplasmic p120ctn regulates the invasive phenotypes of E-cadherin-deficient breast cancer. Am J Pathol 2004;164:2269–2278.
Mastracci TL, Tjan S, Bane AL, et al. E-cadherin alterations in atypical lobular hyperplasia and lobular carcinoma in situ of the breast. Mod Pathol 2005;18:741–751.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esposito, N., Chivukula, M. & Dabbs, D. The ductal phenotypic expression of the E-cadherin/catenin complex in tubulolobular carcinoma of the breast: an immunohistochemical and clinicopathologic study. Mod Pathol 20, 130–138 (2007). https://doi.org/10.1038/modpathol.3800721
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800721
Keywords
This article is cited by
-
E-cadherin to P-cadherin switching in lobular breast cancer with tubular elements
Modern Pathology (2020)
-
Exploring Collagen Parameters in Pure Special Types of Invasive Breast Cancer
Scientific Reports (2019)