Abstract
CDX2 is a homeobox domain-containing transcription factor that is important in the development and differentiation of the intestines. Based on recent studies, CDX2 expression is immunohistochemically detectable in normal colonic enterocytes and is retained in most, but not all, colorectal adenocarcinomas. CDX2 expression has also been documented in a subset of adenocarcinomas arising in the stomach, esophagus and ovary. In this study, we examined CDX2 expression in a series of large tissue microarrays representing 4652 samples of normal and neoplastic tissues. Strong nuclear staining for CDX2 was observed in 97.9% of 140 colonic adenomas, 85.7% of 1109 colonic adenocarcinomas overall and 81.8% of 55 mucinous variants. There was no significant difference in the staining of well-differentiated (96%) and moderately differentiated tumors (90.8%, P=0.18), but poorly differentiated tumors showed reduced overall expression (56.0%, P<0.000001). Correspondingly, there was an inverse correlation between CDX2 expression and tumor stage, with a significant decrease in staining between pT2 and pT3 tumors (95.8 vs 89.0%, P<0.012), and between pT3 and pT4 tumors (89.0 vs 79.8%, P<0.016). Analysis of 140 locally advanced, CDX2-positive colorectal adenocarcinomas coarrayed with their matching lymph node metastases revealed that expression of this marker was retained in 82.1% of the metastases. Consistent with previous reports, CDX2 staining was observed in gastric adenocarcinomas (n=71), more commonly in the intestinal-type than the diffuse-type (28.9 vs 11.5%, P<0.05). Occasional ovarian carcinomas were positive for CDX2, including mucinous (10.5%), endometrioid (9.3%) and serous variants (2%), but expression was either very rare or absent in primary carcinomas of the lung, breast, thyroid, pancreas, liver, gallbladder, kidney, endometrium and urinary bladder. A low frequency of CDX2 expression in pancreatic and biliary carcinomas observed on the microarrays was pursued further by comparing these tumors with ampullary adenocarcinomas on conventional sections. Ampullary adenocarcinomas were more commonly positive for CDX2 (19/24, 79%) than cholangiocarcinomas (1/11, 9%) and pancreatic carcinomas (3/20, 15%). In summary, CDX2 is a sensitive and specific marker for colorectal adenocarcinoma, although its expression is decreased among higher grade and stage tumors, and it is not invariably present in metastases from positive primaries. CDX2 may also be helpful in distinguishing adenocarcinomas of the ampulla from those arising in the pancreas and biliary tree.
Similar content being viewed by others
Main
CDX2 is a homeobox gene that is critical to the development and differentiation of the intestines. A paralogue of the Drosophila caudal gene, CDX2 is essential to body segmentation and tissue patterning at the distal end of the mouse embryo.1, 2, 3 Nuclear expression of CDX2 protein has been detected by immunohistochemistry in fetal and postnatal mice, primarily in the small intestine and proximal colon.4 Similarly, CDX2 has been detected in human fetal intestine at both the mRNA and protein level.5, 6, 7 Conditional expression of CDX2 in an undifferentiated intestinal cell line results in the formation of multicellular structures with demonstrable enterocyte and goblet cell features, supporting a role for CDX2 in intestinal differentiation.8 This likely reflects direct upregulation of transcription from genes such as liver–intestine cadherin and MUC2 by CDX2, as has been shown in other cell lines.9, 10
Direct evidence for a role of CDX2 in intestinal differentiation comes from studies of transgenic mice engineered to express this transcription factor in gastric epithelial cells. Nearly complete intestinalization of the gastric mucosa was observed, including the development of goblet cells expressing acidic-type mucins, enterocyte-like cells expressing alkaline phosphatase and enteroendocrine-type cells.11, 12 In humans, intestinal metaplasia of the stomach and esophagus is consistently accompanied by CDX2 expression.6, 13, 14, 15, 16
The relationship between CDX2 expression and intestinal differentiation suggests that CDX2 may serve as a specific marker for epithelial neoplasms of the gastrointestinal (GI) tract. This is supported by several recently published surveys in which selective staining of colorectal and duodenal adenocarcinomas has been observed using a commercially available monoclonal antibody to CDX2.7, 17, 18 Using the same anti-CDX2 monoclonal antibody and a series of large tissue microarrays, we have confirmed that CDX2 is an excellent marker for adenocarcinomas of the GI tract, and have completed a detailed analysis of its expression across colorectal adenocarcinomas of varying stages and grades.
Materials and methods
Antibodies
A mouse monoclonal IgG1 kappa antibody to CDX2 was purchased from Biogenex (San Ramon, CA, USA). This antibody (clone 7C7/D4) was originally produced against His-tagged, full-length CDX2 that was expressed in Escherichia coli, as detailed in a previous abstract.19 Purified mouse IgG1 (BD Biosciences/Pharmingen, San Diego, CA, USA) was used as a negative control for CDX2 staining. Antibodies to cytokeratin 7 (CK7) and cytokeratin 20 (CK20) were obtained from Dako (Dako Corporation, Carpinteria, CA, USA). Biotinylated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA, USA) and used in the following cocktail for all stains: anti-mouse, IgG (Vector #BA-2000, at 1:400); anti-mouse, IgM (Vector #BA-2020, at 1:200); and anti-rabbit, IgG (Vector #BA-1000, at 1:400). The anti-IgM secondary antibody was not actually necessary for the study, but was included as part of the laboratory's standard cocktail.
Tissues
Microarrays consisting of 0.6 mm cores from formalin-fixed, paraffin-embedded tissues were prepared using a semiautomated tissue arrayer.20 The tissues represented on the arrays were from the archives of the Institute of Pathology, University Hospital, Basel and the Institute of Clinical Pathology, Basel. A total of 10 arrays with between 400 and 580 cores each were used for the study. Each of the arrays contained one core per sampled tissue. Three of the arrays consisted solely of samples of colonic adenoma or adenocarcinoma; information on pathologic grade and stage was available for all three of these arrays. Grade was assigned in accordance with the WHO monograph on gastrointestinal tumors.21 The remaining arrays consisted of a large variety of normal, benign and malignant tissues, including a few additional cases of colonic adenoma and adenocarcinoma. Sections of the arrays, 5 μm thick, were prepared using a tape transfer system (Instrumedics, Inc., Hackensack, NJ, USA). Care was taken to stain each array section in an identical manner. A small percentage of tissue cores was lost from the arrays during staining (average 5.6%, range 0.25–11.5%).
Sections of paraffin-embedded tissues, 5 μm thick, were prepared from blocks selected from the archives of the Department of Pathology, Oregon Health & Science University. The study was conducted according to a protocol approved by the OHSU Institutional Review Board.
Immunohistochemistry
All staining was performed by hand. For CDX2 staining, sections of microarrays or whole tissues were rehydrated and heated in citrate buffer (pH 6.0; Citra, Biogenex) for 40 min in a vegetable steamer (Black & Decker). Slides were allowed to cool to room temperature for 20 min and then incubated with blocking buffer (TBS/0.3% bovine serum albumin (BSA)/0.05% Tween 20) for 15 min. Microarray sections were incubated with CDX2 antibody diluted 1:300 in a phosphate-buffered saline solution (pH 7.1–7.3; Antibody Diluting Buffer, ChemMate, Ventana Medical Systems, Inc.) for 1 h at room temperature. Negative control sections were incubated with 2 μg/ml of mouse IgG1. Following primary antibody incubation, slides were washed three times with TBS/0.05% Tween 20, and then incubated for 30 min at room temperature with a cocktail of biotinylated secondary antibodies in Antibody Dilution Buffer. After additional washes, the slides were incubated in a quench solution consisting of 80% methanol, 1.0 M sodium azide and 30% hydrogen peroxide. The slides were again washed and then incubated for 30 min at room temperature with avidin–biotin complex (Vectastain Elite ABC kit, Vector, prepared as per the manufacturer's instructions). Staining was visualized by incubating for 10 min in DAB solution (K3466, Dako), after which the slides were rinsed in water, counterstained with hematoxylin, dehydrated and coverslipped.
Whole tissue sections were incubated with CDX2 at 1:200 overnight (16 h) at room temperature in a humidified chamber. Pilot stains demonstrated that this longer incubation, which was performed solely as a matter of convenience to the laboratory, had no deleterious effect on the quality of the staining. Incubations with secondary antibody, avidin–biotin complex and DAB were carried out exactly as described above for the microarrays.
For CK7 and CK20 staining, sections were rehydrated and pretreated with a three-step digestion protocol in the following sequence (10 min at room temperature each): (1) 0.12% trypsin (Gibco # 840-7250IL) in TBS/0.1% Tween 20 followed by rinsing with deionized water, (2) 0.01% Pronase (Calbiochem #53702) in TBS followed by rinsing with deionized water, and (3) 0.1% Pepsin (Sigma #P-6887), followed by rinsing with deionized water. Incubations with CK7 (1:1000 in Antibody Diluting Buffer) or CK20 (1:100 in Antibody Diluting Buffer) were performed overnight (approximately 16 h) at room temperature. Incubations with secondary antibody, avidin–biotin complex and DAB were carried out exactly as described above for the microarrays.
Interpretation of Staining
Cores represented on the tissue microarrays and sections of whole tissues were scored in the same manner. Samples that demonstrated unequivocal nuclear staining for CDX2 in at least 10% of representative nuclei were scored as positive. Samples that had nuclear staining of only a few cells (less than 10% of nuclei) were scored as equivocal. These were not included in calculations of overall positive staining. Comparison was made with negative control sections stained with nonspecific IgG1 to be certain that the CDX2 staining was specific, although there was essentially no background with the antibody under the conditions used. Cytoplasmic staining that was occasionally observed in some tissues (eg bile duct epithelium) was ignored. For CK7 and CK20 immunostains, any unequivocal cytoplasmic staining was counted as positive. Statistical analyses were performed by determining a one-sided P-value using the Z-test.22
Results
CDX2 Expression in Normal Tissues
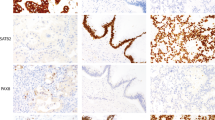
Expression of CDX2 in normal tissues was examined both on tissue microarrays and on selected whole tissue sections. Strong nuclear staining for CDX2 was uniformly observed in the epithelial cells of the small bowel (see Figure 1a) and colon. In addition, there was light staining of epithelial cells lining small ducts in the pancreas, particularly in areas affected by pancreatitis. No nuclear staining was observed in cells lining the main pancreatic duct or distal common bile duct, although cytoplasmic staining was occasionally evident (see Figure 2c). The functional significance of this, if any, is unclear. Other tissues that were negative for CDX2 included esophagus, stomach, liver, lung, thyroid, cervix, endometrium, ovary, spleen, lymph node, skin, brain, peripheral nerve, myocardium, myometrium and skeletal muscle.
CDX2 staining in tissue microarray sections. (a) A colonic adenoma with strong nuclear staining. (b) Four samples of colorectal adenocarcinoma demonstrating varying degrees of staining. (c) Closer view of staining in a moderately differentiated colonic adenocarcinoma. (d) A poorly differentiated colonic adenocarcinoma with patchy nuclear staining. (e) Ovarian carcinoma, endometrioid variant, with positive nuclear staining.
CDX2 staining in whole tissue sections. (a) Metastatic colonic adenocarcinoma in the lung highlighted by strong nuclear staining and some cytoplasmic staining for CDX2. (b) A section of duodenum showing positive staining of the normal mucosa, but no staining of the underlying ductal adenocarcinoma of the pancreas. (c) Ampullary adenocarcinoma with positive nuclear staining; note that the adjacent small pancreatic ducts showed only slight cytoplasmic staining.
CDX2 Expression in Colorectal Neoplasms
Staining results from the tissue microarrays are summarized in Table 1. In brief, strong nuclear staining for CDX2 was nearly universal in samples of colonic adenomas (97.9%; Figure 1a), and was also consistently observed in adenocarcinomas of the colon and rectum (85.7%; Figure 1b and c). Whereas in adenomas the staining was generally restricted to nuclei, cytoplasmic positivity was evident in some of the adenocarcinomas. Among 55 examples of colonic mucinous adenocarcinoma, 45 (81.8%) were positive for CDX2. Single examples of medullary and neuroendocrine carcinoma primary to the colon were negative for CDX2.
As detailed in Table 2, there was a statistically significant inverse correlation between tumor grade and CDX2 staining. Correspondingly, decreased CDX2 staining correlated with increased tumor stage (Table 2). An example of CDX2 staining in a high-grade colonic adenocarcinoma is shown in Figure 1d.
CDX2 Expression in Other Human Tumors
Intestinal-type adenocarcinomas of the stomach were frequently positive for CDX2 (28.9%; Table 1), while staining of diffuse-type carcinomas was statistically less common (11.5%, P<0.05). Occasional cases of mucinous (10.5%) and endometrioid (9.3%) adenocarcinoma of the ovary were positive (Figure 1e). Staining in other types of carcinomas was rare (Table 1).
No CDX2 expression was observed in a wide variety of other cancers represented on the microarrays. These negative tumors included squamous cell carcinoma of the vulva (n=44), penis (n=43), vagina (n=5) and bladder (n=6). Also negative were medullary carcinoma of the breast (n=27), phylloides tumor of the breast (n=13), pheochromocytoma (n=29), paraganglioma (n=10), glomus tumor (n=11), ganglioneuroma (n=7), thyroid adenoma (n=43), medullary (n=9) and anaplastic (n=6) thyroid carcinomas, parathyroid adenoma (n=24) and carcinoma (n=2), adrenal adenoma (n=15) and carcinoma (n=6), renal oncocytoma (n=10), basal cell carcinoma of skin (n=46), Merkel cell carcinoma (n=6), pleomorphic adenoma (n=48), Warthin's tumor (n=30), mucoepidermoid carcinoma (n=5), thymoma (n=24), small-cell carcinoma of the bladder (n=4), adenocarcinoma of the bladder (n=5), sarcomatoid carcinoma of the bladder (n=8), adenocarcinoma of the cervix (n=3), granular cell tumor (n=8), mixed mullerian tumor of ovary (n=6) and adenomatoid tumor (n=19). All central nervous system (CNS) neoplasms were also negative, including glioblastoma multiforme (n=48), schwannoma (n=48), astrocytomas (n=43), meningioma (n=47), oligodendroglioma (n=27), neurofibroma (n=39), craniopharyngioma (n=8), medulloblastoma (n=5), hemangiopericytoma (n=5) and ependymoma (n=12). Likewise, mesenchymal tumors were uniformly CDX2-negative, including leiomyosarcoma (n=49), leiomyoma (n=58), malignant fibrous histiocytoma (n=29), benign histiocytoma (n=30), Kaposi's sarcoma (n=26), liposarcoma (n=26), hemangioma (n=26), rhabdomyosarcoma (n=14), dermatofibrosarcoma protuberans (n=4), synovial sarcoma (n=4), angiosarcoma (n=4), fibrosarcoma (n=9), endometrial stromal sarcoma (n=3) and giant cell tumor of tendon sheath (n=34). A variety of hematologic malignancies were negative for CDX2 as well, including samples of chronic myelogenous leukemia (n=5), Hodgkin's lymphoma (n=55), non-Hodgkin's lymphoma (n=53) and MALT-type lymphoma (n=49).
CDX2 Expression in Metastatic Colorectal Adenocarcinoma
The results from the tissue microarrays indicated that CDX2 is a relatively selective marker for adenocarcinomas deriving from the colon and rectum. To examine this further, a microarray containing matched samples of primary colorectal adenocarcinomas and local lymph node metastases was stained for CDX2. Out of 140 primary tumors that were CDX2 positive, expression was retained in 82.1% of the corresponding lymph node metastases.
CDX2 staining was also examined in distant metastases from 17 well-documented colorectal primaries. The staining was performed on whole tissue sections of these cases, and parallel sections were stained for CK7 and CK20 for comparison (Table 3). As illustrated in Figure 2a, all 17 cases were positive for CDX2 (100%; none was poorly differentiated). Nearly all of the cases were also positive for CK20, but four of them showed coexpression of CK7 (Table 3). Based on this comparison, CDX2 is at least as sensitive a marker for colorectal metastases as the combination of CK20 and CK7.
CDX2 Expression in Pancreatic, Biliary and Ampullary Adenocarcinomas
One striking result of the tissue microarray screening for CDX2 expression was the absence of staining in adenocarcinomas of the pancreas and gallbladder (Table 1). These cancers are frequently included in the differential diagnosis of metastatic adenocarcinoma for which a colorectal primary is suspected. In addition, pancreatic adenocarcinoma can be difficult to distinguish from adenocarcinoma arising in the ampulla of Vater, especially on endoscopic biopsies. To validate the microarray observations, we compared stains for CDX2, CK7 and CK20 in conventional sections of pancreatic adenocarcinoma, cholangiocarcinoma and ampullary carcinoma (Table 4). The latter were defined by accompanying adenomatous epithelium at the ampullary opening or in the ampullary canal. CDX2 expression was much more common in the ampullary adenocarcinomas (19/24, 79%) than in cholangiocarcinomas (1/11, 9%) and pancreatic adenocarcinomas (3/20, 15%). Expression of CK7 and CK20 was more variable among the three tumor types (Table 4).
Discussion
Nuclear transcription factors involved in cellular differentiation provide excellent lineage markers in developmental biology and oncogenesis studies. Two examples that have been widely adopted for clinical use are thyroid transcription factor-1 (TTF-1) and the Wilms' tumor gene product (WT-1). WT-1 is selectively expressed in a subset of pediatric sarcomas as well as cancers arising from mesothelial cells and ovarian surface epithelial cells.23, 24, 25, 26 TTF-1 is a homeodomain-containing protein that serves as an excellent marker for lung and thyroid carcinoma.27 CDX2, another homeodomain-containing transcription factor, is closely linked with intestinal differentiation of gut epithelial cells. In this study, we surveyed the expression of CDX2 in normal and neoplastic human tissues, and examined in detail the relationship between CDX2 staining and tumor grade and stage in colorectal adenocarcinomas.
The anti-CDX2 monoclonal antibody used in our study was the same as that used in three other recently published surveys of this transcription factor. Werling et al7 examined CDX2 expression across 476 samples of human tumors and concluded that it is an excellent marker of adenocarcinomas arising in the GI tract, particularly the duodenum and colon. These authors also reported that a significant fraction of gastric adenocarcinomas, ovarian mucinous carcinomas and primary bladder adenocarcinomas are CDX2 positive. Using tissue microarrays, Moskaluk et al18 analyzed CDX2 staining in 745 samples of human cancer and arrived at similar conclusions. In addition, these authors determined that a significant fraction of carcinoid tumors expresses CDX2, particularly those arising in the hindgut. Finally, Barbareschi et al17 compared CDX2 expression in primary and metastatic tumors found in the lung and concluded that this marker is highly selective for tumors originating from the colon and rectum, but also stains metastases from the stomach and ovary (specifically mucinous carcinomas).
The tissue microarrays used in our study substantially expand the number of tissues screened for CDX2 expression (total of 4652) and the number of colorectal adenocarcinoma samples studied for this marker (1109, approximately five times the sum of tumors published in the previous surveys). We found that CDX2 expression is nearly universal in adenomas and well-differentiated cancers, but is significantly decreased among moderately differentiated and poorly differentiated cancers. This confirms a trend previously observed in much smaller studies by Hinoi et al and Ee et al, although Werling et al noted that '74 of 75 cases of colonic adenocarcinoma were CDX2-positive, independent of tumor grade.7, 28, 29 It is unclear as to why our findings differed from those of Werling et al, but the decrease in CDX2 staining that we observed in higher grade tumors was paralleled by a significant decrease in the staining of higher stage tumors (pT1 and pT2 vs pT3 and pT4).
In the analysis of matched primary and lymph node metastases, we observed a greater than 80% concordance for CDX2 expression. In addition, all 17 colorectal metastases examined by whole sections were CDX2 positive. These findings are entirely consistent with those of Werling et al7 and Barbareschi et al,17 and support the use of this marker in the evaluation of metastatic adenocarcinoma of unknown primary.
CDX2 expression has been documented in gastric adenocarcinoma by several different groups.7, 13, 15, 16, 17, 18, 30 Our results are entirely consistent with these studies in that CDX2 staining was observed in only a subset of gastric cancers and significantly favored the intestinal-type tumors over the diffuse variants.
With regard to CDX2 expression in cholangiocarcinomas and pancreatic carcinomas, there appears to be somewhat less agreement. Werling et al7 reported that 25% (4/16) of cholangiocarcinomas, including gallbladder primaries, showed significant (2+ or 3+) CDX2 staining. Moskaluk et al18 examined 23 gallbladder and other cholangiocarcinomas and observed staining in 11 (48%), although nine of these were only 1+ positive. In contrast, we saw no staining in 25 arrayed gallbladder tumors and very little staining in whole sections of cholangiocarcinoma (1/11, 9%). Likewise, we observed CDX2 expression in a smaller fraction of pancreatic ductal carcinomas (3/20 of whole sections, 0/50 of arrayed samples), than both Werling et al (7/22, 32%) and Moskaluk et al (8/24, 33%). While the reason for these differences is unclear, we agree with Werling et al7 that diffuse, strong nuclear staining for CDX2 is more characteristic of a small bowel or colorectal adenocarcinoma than a pancreatic carcinoma. Consistent with this view, we found that CDX2 expression was common in ampullary carcinomas (19/24, 79%), confirming a trend observed by Moskaluk et al18 (4/6, 67%).
In summary, CDX2 is a sensitive and specific marker for colorectal adenocarcinoma, although its expression is decreased among higher grade and stage tumors, and it is not invariably present in metastases from positive primaries. CDX2 may also be helpful in distinguishing adenocarcinomas of the ampulla from those arising in the pancreas and biliary tree.
References
Chawengsaksophak K, James R, Hammond VE, et al. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 1997;386:84–87.
Drummond F, Putt W, Fox M, et al. Cloning and chromosome assignment of the human CDX2 gene. Ann Hum Genet 1997;61(Part 5): 393–400.
van den AE, Forlani S, Chawengsaksophak K, et al. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development 2002;129:2181–2193.
Silberg DG, Swain GP, Suh ER, et al. Cdx1 and Cdx2 expression during intestinal development. Gastroenterology 2000;119:961–971.
Mallo GV, Rechreche H, Frigerio JM, et al. Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and Cdx2 homeobox. Down-regulation of Cdx1 and Cdx2 mRNA expression during colorectal carcinogenesis. Int J Cancer 1997;74:35–44.
Mizoshita T, Inada K, Tsukamoto T, et al. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa—with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer 2001;4:185–191.
Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol 2003;27:303–310.
Suh E, Traber PG . An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 1996;16:619–625.
Hinoi T, Lucas PC, Kuick R, et al. CDX2 regulates liver–intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology 2002;123:1565–1577.
Yamamoto H, Bai YQ, Yuasa Y . Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun 2003;300:813–818.
Mutoh H, Hakamata Y, Sato K, et al. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun 2002;294:470–479.
Silberg DG, Sullivan J, Kang E, et al. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology 2002;122:689–696.
Almeida R, Silva E, Santos-Silva F, et al. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol 2003;199:36–40.
Phillips RW, Frierson Jr HF, Moskaluk CA . Cdx2 as a marker of epithelial intestinal differentiation in the esophagus. Am J Surg Pathol 2003;27:1442–1447.
Satoh K, Mutoh H, Eda A, et al. Aberrant expression of CDX2 in the gastric mucosa with and without intestinal metaplasia: effect of eradication of Helicobacter pylori. Helicobacter 2002;7:192–198.
Seno H, Oshima M, Taniguchi MA, et al. CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: prognostic implications. Int J Oncol 2002;21:769–774.
Barbareschi M, Murer B, Colby TV, et al. CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastases to the lungs. Am J Surg Pathol 2003;27:141–149.
Moskaluk CA, Zhang H, Powell SM, et al. Cdx2 protein expression in normal and malignant human tissues: an immunohistochemical survey using tissue microarrays. Mod Pathol 2003;16:913–919.
Wang J, Nguyen JT, Yu G, et al. Development of a monoclonal antibody directed against CDX-2, an intestinal epithelial cell lineage marker. Abstract, National Society for Histotechnology 2000.
Torhorst J, Bucher C, Kononen J, et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol 2001;159:2249–2256.
Hamilton SR, Aaltonen LA . Tumours of the Digestive Tract. WHO/IARC Press: Lyon, France, 2000, pp 110–111.
Ramsey FL, Schafer DW . The Statistical Sleuth. International Thomson Publishing Company: Albany, NY, 1997.
Charles AK, Moore IE, Berry PJ . Immunohistochemical detection of the Wilms' tumour gene WT1 in desmoplastic small round cell tumour. Histopathology 1997;30:312–314.
Hill DA, Pfeifer JD, Marley EF, et al. WT1 staining reliably differentiates desmoplastic small round cell tumor from Ewing sarcoma/primitive neuroectodermal tumor. An immunohistochemical and molecular diagnostic study. Am J Clin Pathol 2000;114:345–353.
Ramani P, Cowell JK . The expression pattern of Wilms' tumour gene (WT1) product in normal tissues and paediatric renal tumours. J Pathol 1996;179:162–168.
Shimizu M, Toki T, Takagi Y, et al. Immunohistochemical detection of the Wilms' tumor gene (WT1) in epithelial ovarian tumors. Int J Gynecol Pathol 2000;19:158–163.
Ordonez NG . Thyroid transcription factor-1 is a marker of lung and thyroid carcinomas. Adv Anat Pathol 2000;7:123–127.
Ee HC, Erler T, Bhathal PS, et al. Cdx-2 homeodomain protein expression in human and rat colorectal adenoma and carcinoma. Am J Pathol 1995;147:586–592.
Hinoi T, Tani M, Lucas PC, et al. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol 2001;159:2239–2248.
Bai YQ, Yamamoto H, Akiyama Y, et al. Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett 2002;176:47–55.
Acknowledgements
We are indebted to Linda Jauron-Mills and Donald Anderson for their expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaimaktchiev, V., Terracciano, L., Tornillo, L. et al. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol 17, 1392–1399 (2004). https://doi.org/10.1038/modpathol.3800205
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800205
Keywords
This article is cited by
-
Hidden colon adenocarcinoma diagnosed from mouth metastasis: case report and literature review
World Journal of Surgical Oncology (2023)
-
Protective role of estrogen through G-protein coupled receptor 30 in a colitis mouse model
Histochemistry and Cell Biology (2023)
-
Loss of SATB2 and CDX2 expression is associated with DNA mismatch repair protein deficiency and BRAF mutation in colorectal cancer
Medical Molecular Morphology (2023)
-
Comprehensive clinicopathologic, molecular, and immunologic characterization of colorectal carcinomas with loss of three intestinal markers, CDX2, SATB2, and KRT20
Virchows Archiv (2022)
-
Metastatic colon cancer of the small intestine diagnosed using genetic analysis: a case report
Diagnostic Pathology (2020)