Abstract
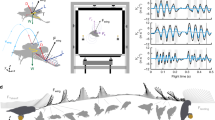
Aerodynamic theory predicts that the power required for an animal to fly over a range of speeds is represented by a ‘U’-shaped curve, with the greatest power required at the slowest and fastest speeds, and minimum power at an intermediate speed1,2,3,4,5,6. Tests of these predictions, based on oxygen consumption measurements of metabolic power in birds7,8,9,10,11,12 and insects13, support a different interpretation, generating either flat or ‘J’-shaped power profiles, implying little additional demand between hovering and intermediate flight speeds14. However, respirometric techniques represent only an indirect assessment of the mechanical power requirements of flight and no previous avian study has investigated an animal's full range of attainable level flight speeds. Here we present data from in vivo bone-strain measurements of pectoralis muscle force coupled with wing kinematics in black-billed magpies (Pica pica ), which we use to calculate mechanical power directly. As these birds flew over their full range of speeds, we offer a complete profile of mechanical power output during level flapping flight for this species. Values of mechanical power output are statistically indistinguishable (that is, the power curve is flat) over most forward-flight speeds but are significantly higher during hovering and flight at very low speeds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tucker, V. A. Bird metabolism during flight: evaluation of a theory. J. Exp. Biol. 58, 689–709 (1973).
Pennycuick, C. J. Power requirements for horizontal flight in the pigeon Columba livia. J. Exp. Biol. 49, 527–555 (1968).
Pennycuick, C. J. in Avian Biology (eds. Farner, D. S. & King, J. R.) 5, 1–75 (1975).
Pennycuick, C. J. Bird Flight Performance (Univ. Press, Oxford, (1989)).
Rayner, J. M. V. Anew approach to animal flight mechanics. J. Exp. Biol. 80, 17–54 (1979).
Norberg, U. M. Vertebrate Flight (Springer, Berlin, (1990)).
Tucker, V. A. Respiratory exchange and evaporative water loss in the flying budgerigar. J. Exp. Biol. 48, 67–87 (1968).
Tucker, V. A. Metabolism during flight in the laughing gull, Larus atricilla. Am. J. Physiol. 222, 237–245 (1972).
Hudson, D. M. & Bernstein, M. H. Gas exchange and energy cost of flight in the white-necked raven, Corvus cryptoleucus. J. Exp. Biol. 103, 121–130 (1983).
Berger, M., Hart, O. Z. & Roy, J. S. Respiration, oxygen consumption and heart rate in some birds during rest and flight. Z. Vergl. Physiol. 66, 201–214 (1970).
Torre-Bueno, J. R. & LaRochelle, J. The metabolic cost of flight in unrestrained birds. J. Exp. Biol. 75, 223–229 (1978).
Rothe, H.-J., Biesel, W. & Nachtigall, W. Pigeon flight in a wind tunnel. II. Gas exchange and power requirements. J. Comp. Physiol. B 157, 99–109 (1987).
Ellington, C. P., Machin, K. I. & Casey, T. M. Oxygen consumption of bumblebees in forward flight. Nature 347, 472–473 (1990).
Ellington, C. P. Limitations on animal flight performance. J. Exp. Biol. 160, 71–91 (1991).
Dial, K. P. & Biewener, A. A. Pectoralis muscle force and power output during different modes of flight in pigeons (Columba livia ). J. Exp. Biol. 176, 31–54 (1993).
Tobalske, B. W. & Dial, K. P. Neuromuscular control and kinematics of intermittent flight in budgerigars (Melopsittacus undulatus ). J. Exp. Biol. 187, 1–13 (1994).
Josephson, R. K. The mechanical power output of a tettigonid wing muscle during singing and flight. J. Exp. Biol. 117, 357–368 (1985).
Tobalske, B. W. & Dial, K. P. Flight kinematics of black-billed magpies and pigeons over a wide range of speeds. J. Exp. Biol. 199, 263–280 (1996).
Biewener, A. A. & Dial, K. P. In vivo strain in the pigeon humerus during flight. J. Morph. 225, 61–75 (1995).
Hedenstrom, A. & T. Alerstam. Skylark optimal flight speeds for flying nowhere and somewhere. Behav. Ecology 7, 121–126.
Liechti, F., Ehrich, D. & Bruderer, B. Flight behaviour of white storks Ciconia ciconia on their migration over Southern Israel. Ardea 84, 3–13 (1997).
Biewener, A. A., Dial, K. P. & Goslow, G. E. J Pectoralis muscle force and power output during flight in the starling. J. Exp. Biol. 164, 1–18 (1992).
Dial, K. P. Activity patterns of the wing muscles in the pigeon (Columba livia ) during different modes of flight. J. Exp. Zool. 262, 357–373 (1992).
Dial, K. P. Avian forelimb muscles and nonsteady flight: can birds fly without using the muscles in their wings? Auk 109, 874–885 (1992).
Van Den Berg, C. & Rayner, J. M. V. The moment of inertia of bird wings and the inertial power requirement for flapping flight. J. exp. Biol. 198, 1655–1664 (1995).
Scholey, K. D. Evelopments in Vertebrate Flight: Climbing and Gliding of Mammals and Reptiles and the Flapping Flight of Birds. thesis, Univ. Bristol (1983).
Acknowledgements
We thank F. A. Jenkins Jr for his thoughtful input on previous drafts of this manuscript; M. LaBarbera, J. M. Marzluff, D. Fawcett and D. F. Boggs for their comments; and J. Gilpin for making the force-transducer used to calibrate the DPC strain recordings. Supported by grants from the NSF to K.P.D. and A.A.B.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dial, K., Biewener, A., Tobalske, B. et al. Mechanical power output of bird flight. Nature 390, 67–70 (1997). https://doi.org/10.1038/36330
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/36330
This article is cited by
-
Investigation of the rate-mediated form-function relationship in biological puncture
Scientific Reports (2023)
-
On the role of tail in stability and energetic cost of bird flapping flight
Scientific Reports (2022)
-
Convergent genomic signatures of flight loss in birds suggest a switch of main fuel
Nature Communications (2019)
-
Birds repurpose the role of drag and lift to take off and land
Nature Communications (2019)
-
When three per cent may not be three per cent; device-equipped seabirds experience variable flight constraints
Marine Biology (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.