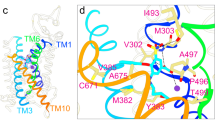
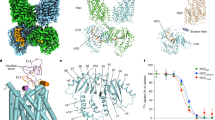
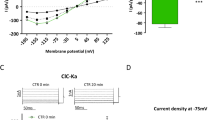
Abstract
Renal salt loss in Bartter's syndrome is caused by impaired transepithelial transport in the loop of Henle. Sodium chloride is taken up apically by the combined activity of NKCC2 (Na+-K--2Cl- cotransporters) and ROMK potassium channels. Chloride ions exit from the cell through basolateral ClC-Kb chloride channels. Mutations in the three corresponding genes have been identified1,2,3 that correspond to Bartter's syndrome types 1–3. The gene4 encoding the integral membrane protein barttin is mutated in a form of Bartter's syndrome that is associated with congenital deafness and renal failure. Here we show that barttin acts as an essential β-subunit for ClC-Ka and ClC-Kb chloride channels, with which it colocalizes in basolateral membranes of renal tubules and of potassium-secreting epithelia of the inner ear. Disease-causing mutations in either ClC-Kb or barttin compromise currents through heteromeric channels. Currents can be stimulated further by mutating a proline-tyrosine (PY) motif on barttin. This work describes the first known β-subunit for CLC chloride channels and reveals that heteromers formed by ClC-K and barttin are crucial for renal salt reabsorption and potassium recycling in the inner ear5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Simon, D. B. et al. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nature Genet. 13, 183–188 (1996).
Simon, D. B. et al. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nature Genet. 14, 152–156 (1996).
Simon, D. B. et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nature Genet. 17, 171–178 (1997).
Birkenhäger, R. et al. Mutations of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nature Genet. 29, 310–314 (2001).
Jentsch, T. J. Neuronal KCNQ channel: physiology and role in disease. Nature Rev. Neurosci. 1, 21–30 (2000).
Kieferle, S., Fong, P., Bens, M., Vandewalle, A. & Jentsch, T. J. Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc. Natl Acad. Sci. USA 91, 6943–6947 (1994).
Vandewalle, A. et al. Localization and induction by dehydration of ClC-K chloride channels in the rat kidney. Am. J. Physiol. 272, F678–F688 (1997).
Kobayashi, K., Uchida, S., Mizutani, S., Sasaki, S. & Marumo, F. Intrarenal and cellular localization of ClC-K2 protein in the mouse kidney. J. Am. Soc. Nephrol. 12, 1327–1334 (2001).
Uchida, S. et al. Localization and functional characterization of rat kidney-specific chloride channel, ClC-K1. J. Clin. Invest. 95, 104–113 (1995).
Matsumura, Y. et al. Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nature Genet. 21, 95–98 (1999).
Uchida, S. et al. Molecular cloning of a chloride channel that is regulated by dehydration and expressed predominantly in kidney medulla. J. Biol. Chem. 268, 3821–3824 (1993); erratum J. Biol. Chem. 269, 19192 (1994).
Waldegger, S. & Jentsch, T. J. Functional and structural analysis of ClC-K chloride channels involved in renal disease. J. Biol. Chem. 275, 24527–24533 (2000).
Zerangue, N., Schwappach, B., Jan, Y. N. & Jan, L. Y. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22, 537–548 (1999).
Schwake, M., Friedrich, T. & Jentsch, T. J. An internalization signal in ClC-5, an endosomal Cl--channel mutated in Dent's disease. J. Biol. Chem. 276, 12049–12054 (2001).
Jentsch, T. J., Friedrich, T., Schriever, A. & Yamada, H. The CLC chloride channel family. Pflügers Arch. Eur. J. Physiol. 437, 783–795 (1999).
Staub, O. et al. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 15, 2371–2380 (1996).
Schild, L. et al. Identification of a PY motif in the epithelial Na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J. 15, 2381–2387 (1996).
Staub, O. et al. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 16, 6325–6336 (1997).
Konrad, M. et al. Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J. Am. Soc. Nephrol. 11, 1449–1459 (2000).
Alper, S. L., Natale, J., Lodish, H. F. & Brown, D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc. Natl Acad. Sci. USA 86, 5429–5433 (1989).
Nielsen, S., DiGiovanni, S. R., Christensen, E. I., Knepper, M. A. & Harris, H. W. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc. Natl Acad. Sci. USA 90, 11663–11667 (1993).
Ando, M. & Takeuchi, S. mRNA encoding ‘ClC-K1, a kidney Cl- channel’ is expressed in marginal cells of the stria vascularis of rat cochlea: its possible contribution to Cl- currents. Neurosci. Lett. 284, 171–174 (2000).
Baloh, R. W. Vertigo. Lancet 352, 1841–1846 (1998).
Jeck, N. et al. Hypokalemic salt-losing tubulopathy with chronic renal failure and sensorineural deafness. Pediatrics 108, E5 (2001).
Delpire, E., Lu, J., England, R., Dull, C. & Thorne, T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nature Genet. 22, 192–195 (1999).
Dixon, M. J. et al. Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum. Mol. Genet. 8, 1579–1584 (1999).
Neyroud, N. et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nature Genet. 15, 186–189 (1997).
Schulze-Bahr, E. et al. KCNE1 mutations cause Jervell and Lange-Nielsen syndrome. Nature Genet. 17, 267–268 (1997).
Dedek, K. & Waldegger, S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflügers Arch. Eur. J. Physiol. 442, 896–902 (2001).
Acknowledgements
We thank S. Alper for the AE1 antibody, M. Knepper for the aquaporin-2 antibody, M. Knipper for advice on inner ear immunohistochemistry, and J. Enderich and M. Kolster for technical assistance. R.E. is a recipient of a Marie Curie Human Potential Fellowship of the European Union, and F.H. is a Heisenberg scholar of the Deutsche Forschungsgemeinschaft (DFG). This work was supported by grants from the DFG, the Fonds der Chemischen Industrie, and the Prix Louis Jeantet de Médecine to T.J.J., and from the Federal State of Baden-Württemberg to F.H.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information

Figure A. Functional characterisation of CIC-K/barttin channels expressed in transfected tsA201 cells
(JPG 137.85 KB)

Figure B. Topology of barttin
(JPG 11.5 KB)
The first hydrophobic region of barttin may span the membrane or may act as a cleavable signal peptide. The latter possibility is suggested by the program SMART (http://smart.embl-heidelberg.de). To distinguish between these possibilities, we epitope-tagged barttin at either end. This did not affect its ability to elicit currents with ClC-Ka. When these constructs were expressed in COS cells with or without ClC-Ka (Ka) and analysed by Western blotting using an antibody against the epitope, bands corresponding in size to barttin were stained with comparable intensities irrespective of the position of the epitope (Fig. B). Mock-transfected COS cells (Ctrl) and ClC-Ka transfected COS cells were used as a controls (left two lanes). This argues against an amino-terminal cleavage, and supports a model in which barttin has two transmembrane spanning segments in its amino-terminal end.

Figure C. RT-PCR detection of ClC-K1 and ClC-K2 in the cochlea
(JPG 15.24 KB)
To examine whether ClC-K1 and ClC-K2 is expressed in the cochlea, we performed RT-PCR experiments on RNA obtained from mouse cochlea. The primers used could differentiate between the highly homologous (~90% identity) ClC-K1 and ClC-K2 mRNAs. Using PCR conditions and primers described in reference 24, we observed bands of the correct sizes in samples containing cochlear RNA, but not in controls (Fig. C). Thus, both ClC-K1 and ClC-K2 are expressed in the cochlea. Because immunofluorescence indicates that ClC-K channels are only present in the stria vascularis, this strongly suggests that these cells express both isoforms.

Figure D. Antibodies against barttin
(JPG 131.28 KB)
Rights and permissions
About this article
Cite this article
Estévez, R., Boettger, T., Stein, V. et al. Barttin is a Cl- channel β-subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature 414, 558–561 (2001). https://doi.org/10.1038/35107099
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35107099
This article is cited by
-
Anoctamin 1 controls bone resorption by coupling Cl− channel activation with RANKL-RANK signaling transduction
Nature Communications (2022)
-
Development of the stria vascularis in the common marmoset, a primate model
Scientific Reports (2022)
-
Clues and new evidences in arterial hypertension: unmasking the role of the chloride anion
Pflügers Archiv - European Journal of Physiology (2022)
-
Genetic diagnosis and treatment of hereditary renal tubular disease with hypokalemia and alkalosis
Journal of Nephrology (2022)
-
Antenatal Indomethacin Use Altering the Initial Presentation of Type 4A Bartter Syndrome
Indian Journal of Pediatrics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.