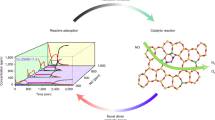
Abstract
Although many enzymes can readily and selectively use oxygen in water—the most familiar and attractive of all oxidants and solvents, respectively—the design of synthetic catalysts for selective water-based oxidation processes utilizing molecular oxygen1,2,3,4 remains a daunting task5,6. Particularly problematic is the fact that oxidation of substrates by O2 involves radical chemistry, which is intrinsically non-selective and difficult to control. In addition, metallo-organic catalysts are inherently susceptible to degradation5 by oxygen-based radicals, while their transition-metal-ion active sites often react with water to give insoluble, and thus inactive, oxides or hydroxides7. Furthermore, pH control is often required to avoid acid or base degradation of organic substrates or products. Unlike metallo-organic catalysts, polyoxometalate anions are oxidatively stable and are reversible oxidants8,9 for use with O2 (refs 8,9,10). Here we show how thermodynamically controlled self-assembly of an equilibrated ensemble of polyoxometalates, with the heteropolytungstate anion11,12 [AIVVW11O40]6- as its main component, imparts both stability in water and internal pH-management. Designed to operate at near-neutral pH, this system facilitates a two-step O2-based process for the selective delignification of wood (lignocellulose) fibres. By directly monitoring the central Al atom, we show that equilibration reactions typical of polyoxometalate anions13,14 keep the pH of the system near 7 during both process steps.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Groves, J. T. & Quinn, R. Aerobic epoxidation of olefins with ruthenium porphyrin catalysts. J. Am. Chem. Soc. 107, 5790–5792 (1985).
Neumann, R. & Dahan, M. Molecular oxygen activation: A ruthenium substituted polyoxometalate as an inorganic dioxygenase. Nature 388, 353–355 (1997).
Weiner, H. & Finke, R. G. An all-inorganic, polyoxometalate-based catechol dioxygenase that exhibits >100,000 catalytic turnovers. J. Am. Chem. Soc. 121, 9831–9842 (1999).
Döbler, C., Mehltretter, G. M., Sundermeier, U. & Beller, M. Osmium-catalyzed dihydroxylation of olefins using dioxygen or air as the terminal oxidant. J. Am. Chem. Soc. 122, 10289–10297 (2000).
Hill, C. L. & Weinstock, I. A. Homogeneous catalysis: On the trail of dioxygen activation. Nature 388, 332–333 (1997).
Hill, C. L. Homogeneous catalysis: Controlled green oxidation. Nature 401, 436–437 (1999).
Baes, C. F. Jr & Mesmer, R. E. The Hydrolysis of Cations (Wiley & Sons, New York, 1976).
Hill, C. L. Special thematic issue on polyoxometalates. Chem. Rev. 98, 1–390 (1998).
Neumann, R. Polyoxometalate complexes in organic oxidation chemistry. Prog. Inorg. Chem. 47, 317–370 (1998).
Weinstock, I. A. Homogeneous-phase electron-transfer reactions of polyoxometalates. Chem. Rev. 98, 113–170 (1998).
Pope, M. T. & Müller, A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines. Angew. Chem. Int. Edn Engl. 30, 34–48 (1991).
Weinstock, I. A., Cowan, J. J., Barbuzzi, E. M. G., Zeng, H. & Hill, C. L. Equilibria between α and β isomers of keggin heteropolytungstates. J. Am. Chem. Soc. 121, 4608–4617 (1999).
Andersson, I., Hastings, J. J., Howarth, O. W. & Pettersson, L. Aqueous tungstovanadate equilibria. J. Chem. Soc. Dalton Trans. 2705–2711 (1996)
Grate, J. H., Hamm, D. R. & Mahajan, S. in Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity (eds Pope, M. T. & Müller, A.) 281–306 (Kluwer, Dordrecht, 1994).
Sjostrom, E. Wood Chemistry: Fundamentals and Applications (Academic, San Diego, 1992).
Hiskia, A. & Papaconstantinou, E. Photocatalytic oxidation of organic compounds by polyoxometalates of molybdenum and tungsten. Catalyst regeneration by dioxygen. Inorg. Chem. 31, 163–167 (1992).
Duncan, D. C. & Hill, C. L. Mechanism of reaction of reduced polyoxometalates with O2 evaluated by 17O NMR. J. Am. Chem. Soc. 119, 243–244 (1997).
Birchmeier, M. J., Hill, C. G. J., Houtman, C. J., Atalla, R. H. & Weinstock, I. A. Enhanced wet air oxidation: synergistic rate acceleration upon effluent recirculation. Ind. Eng. Chem. Res. 39, 55–64 (2000).
Weinstock, I. A., Atalla, R. H., Reiner, R. S., Houtman, C. J. & Hill, C. L. Selective transition-metal catalysis of oxygen delignification using water-soluble salts of polyoxometalate (POM) anions. Part I. Chemical principles and process concepts. Holzforschung 52, 304–310 (1998).
Akitt, J. W. & Farthing, A. Aluminium-27 nuclear magnetic resonance studies of heteropolyanions containing aluminium as heteroatom. J. Chem. Soc. Dalton Trans. 1615–1616 (1981).
Akitt, J. W. in Modern NMR Techniques and their Applications in Chemistry (eds Popov, A. I. & Hallenga, K.) Ch. 7, 427–483 (Dekker, New York, 1991).
Pope, M. T. Heteropoly and Isopoly Oxometalates (Springer, Berlin, 1983).
TAPPI Test Methods 1991 (Tappi Press, Atlanta, Georgia, 1991).
Weinstock, I. et al. Selective transition-metal catalysis of oxygen delignification using water-soluble salts of polyoxometalate (POM) anions. Part II. Reactions of alpha-[SiVW11O40]5- with phenolic lignin-model compounds. Holzforschung 52, 311–318 (1998).
Carrier, X., de la Caillerie, J. B. d. E., Lambert, J. F. & Che, M. The support as a chemical reagent in the preparation of WOx/gamma-Al2O3 catalysts: Formation and deposition of aluminotungstic heteropolyanions. J. Am. Chem. Soc. 121, 3377–3381 (1999).
Dence, C. W. & Reeve, D. W. (eds) Pulp Bleaching: Principles and Practice (Tappi Press, Atlanta, Georgia, 1996).
Grigoriev, V. A., Hill, C. L. & Weinstock, I. A. Role of alkali metal cation size in the energy and rate of electron-transfer to solvent-separated 1:1 [(M+)(Acceptor)] (M+ = Li+, Na+, K+) ion pairs. J. Am. Chem. Soc. 123, 5292–5307 (2001).
Myron, D. R., Givand, S. H. & Nielsen, F. H. Vanadium content of selected foods as determined by flameless atomic absorption spectroscopy. J. Agric. Food Chem. 25, 297–299 (1977).
Kletzin, A. & Adams, M. W. W. Tungsten in biological systems. FEMS Microbiol. Rev. 18, 5–63 (1996).
Pettersson, L. in Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity (eds Pope, M. T. & Müller, A.) 27–40 (Kluwer, Dordrecht, 1994).
Acknowledgements
I.A.W. is a visiting scientist at the Department of Chemistry, Emory University, but based at the Forest Products Laboratory. We thank W. E. Bowerman, H. Zeng, S. Ralph and L. L. Landucci for technical assistance; we also acknowledge the contributions of R. H. Atalla, M. J. Birchmeier, C. G. Hill Jr, C. J. Houtman, S. E. Reichel and E. L. Springer. This work was supported by the DOE, NSF and member companies of the USDA Forest Service, Polyoxometalate Bleaching Consortium.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Weinstock, I., Barbuzzi, E., Wemple, M. et al. Equilibrating metal-oxide cluster ensembles for oxidation reactions using oxygen in water. Nature 414, 191–195 (2001). https://doi.org/10.1038/35102545
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35102545
This article is cited by
-
N-formylation of amines using methanol as a potential formyl carrier by a reusable chromium catalyst
Communications Chemistry (2019)
-
Design of an inherently-stable water oxidation catalyst
Nature Communications (2018)
-
Advanced redox flow fuel cell using ferric chloride as main catalyst for complete conversion from carbohydrates to electricity
Scientific Reports (2017)
-
Synthesis, Characterization and Catalytic Activity of a Tungsten(VI) Amino Triphenolate Complex
Catalysis Letters (2017)
-
Solar-induced direct biomass-to-electricity hybrid fuel cell using polyoxometalates as photocatalyst and charge carrier
Nature Communications (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.