Abstract
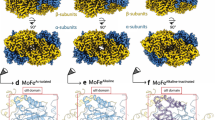
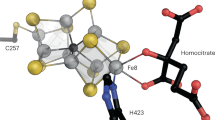
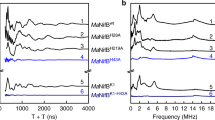
TWO components constitute Mo-dependent nitrogenase (EC 1.18.6.1)— the Fe protein (a homodimer encoded by nifH) and the MoFe protein (an α2β2 tetramer encoded by nifDK). The MoFe protein provides the substrate-binding site1–3 and probably coná-tains six prosthetic groups of two types—four Fe-S centres and two Fe- and Mo-containing cofactors4,5. To determine the distribution and catalytic function of these metalloclusters, we6,7 and others8 are attempting to change the catalytic and spectroscopic features of nitrogenase by substituting specific amino acids targeted as potential metallocluster ligands, particularly those to the FeMo-cofactor, which is responsible for the biologically unique electron paramagnetic resonance signal (S =3/2) of nitrogenase9,10, and is believed to be the N2-reducing site11. Here we describe mutant strains of Azotobacter vinelandii that have single a mi no-acid substitutions within the MoFe protein α-subunit. These substitutions alter both substrate-reduction properties and the unique electron paramagnetic resonance signal, indicating that the FeMo-cofactor is associated with both the α-subunit and the substrate-reducing site.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Orme-Johnson, W. H. et al. Proc. natn. Acad. Sci. U.S.A. 69, 3142–3145 (1972).
Mortenson, L. E., Zumft, W. G. & Palmer, G. Biochim. biophys. Acta 292, 422–435 (1973).
Smith, B. E., Lowe, D. J. & Bray, R. C. Biochem. J. 135, 331–341 (1973).
Smith, B. E. & Lang, G. Biochem. J. 137, 169–180 (1974).
Zimmerman, R. et al. Biochim. biophys. Acta 537, 185–207 (1978).
Brigle, K. E. et al. Proc. natn. Acad. Sci. U.S.A. 84, 7066–7069 (1987).
Dean, D. R., Brigle, K. E., May, H. D. & Newton, W. E. in Nitrogen Fixation: Hundred Years After (eds Bothe, H., de Bruijn, F. J. & Newton, W. E.) 107–113 (Fischer, Stuttgart, 1988).
Kent, H. M., Ionnidis, I., Gormal, C., Smith, B. E. & Buck, M. Biochem. J. 264, 257–264 (1989).
Shah, V. K. & Brill, W. J. Proc. natn. Acad. Sci. U.S.A. 74, 3249–3253 (1977).
Rawlings, J. et al. J. biol. Chem. 253, 1001–1004 (1978).
Hawkes, T. R., McLean, P. A. & Smith, B. E. Biochem. J. 217, 317–321 (1984).
Telser, J. et al. FEBS lett. 214, 117–121 (1987).
Yang, S.-S. et al. J. biol. Chem. 257, 8042–8048 (1982).
Thomann, H. et al. J. Am. chem. Soc. 109, 7913–7914 (1987).
Walters, M. A., Chapman, S. K. & Orme-Johnson, W. H. Polyhedron 5, 561–565 (1986).
Brigle, K. E., Weiss, M. C., Newton, W. E. & Dean, D. R. J. Bact. 169, 1547–1553 (1987).
Brigle, K. E. & Dean, D. R. Nucleic Acids Res. 16, 5214 (1988).
Setterquist, R. et al. Nucleic Acids Res. 16, 5215 (1988).
Arnold, W., Rump, A., Klipp, W., Priefer, U. B. & Puhler, A. J. molec. Biol. 230, 715–738 (1988).
Moreno-Vivian, C., Schmel, M., Masepohl, B., Arnold, W. & Klipp, W. Molec. gen. Genet. 216, 353–363 (1989).
Wang, S.-Z., Chen, J.-S. & Johnson, J. L. Nucleic Acids Res. 17, 3299 (1989).
Paustian, T. D., Shah, V. K. & Roberts, G. P. Proc. natn. Acad. Sci. U.S.A. 86, 6082–6086 (1989).
Dilworth, M. J., EAdy, R. R., Robson, R. L. & Miller, R. W. Nature 327, 167–168 (1987).
Jacobson, M. R., Premakumar, R. & Bishop, P. E. J. Bact. 167, 480–486 (1986).
Eady, R. R., Robson, R. L., Richardson, T. H., Miller, R. W. & Hawkins, M. Biochem. J. 244, 197–207 (1987).
Hales, G. J., Case, E. E., Morningstar, J. E., Dzeda, M. F. & Mauterer, L. A. Biochemistry 25, 7251–7255 (1986).
Brigle, K. E., Newton, W. E. & Dean, D. R. Gene 37, 37–44 (1985).
Burgess, B. K., Jacobs, D. J. & Stiefel, E. I. Biochim. biophys. Acta 614, 196–209 (1980).
Gornall, A. G., Bardawell, C. J. & David, M. M. J. biol. Chem. 177, 751–766 (1948).
Jacobson, M. R. et al. J. Bact. 171, 1017–1027 (1989).
Howard, K. S., Hales, B. J. & Socolofsky, M. D. Biochim. biophys. Acta 812, 575–585 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scott, D., May, H., Newton, W. et al. Role for the nitrogenase MoFe protein α-subunit in FeMo-cofactor binding and catalysis. Nature 343, 188–190 (1990). https://doi.org/10.1038/343188a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/343188a0
This article is cited by
-
Catalysts for nitrogen reduction to ammonia
Nature Catalysis (2018)
-
Mutagenesis at α-423Ile of MoFe protein reduces the catalytic activity of nitrogenase in Klebsiella oxytoca
Chinese Science Bulletin (2014)
-
Variant MoFe proteins of Azotobacter vinelandii: effects of carbon monoxide on electron paramagnetic resonance spectra generated during enzyme turnover
JBIC Journal of Biological Inorganic Chemistry (2005)
-
Construction and characterization of double mutants in nitrogenase ofKlebsiella pneumoniae
Chinese Science Bulletin (2004)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.