Abstract
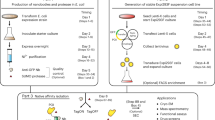
Lentiviral vectors pseudotyped with vesicular stomatitis virus glycoprotein (VSV-G) are emerging as the vectors of choice for in vitro and in vivo gene therapy studies. However, the current method for harvesting lentivectors relies upon ultracentrifugation at 50000 g for 2 h. At this ultra-high speed, rotors currently in use generally have small volume capacity. Therefore, preparations of large volumes of high-titre vectors are time-consuming and laborious to perform. In the present study, viral vector supernatant harvests from vector-producing cells (VPCs) were pre-treated with various amounts of poly-L-lysine (PLL) and concentrated by low speed centrifugation. Optimal conditions were established when 0.005% of PLL (w/v) was added to vector supernatant harvests, followed by incubation for 30 min and centrifugation at 10000 g for 2 h at 4°C. Direct comparison with ultracentrifugation demonstrated that the new method consistently produced larger volumes (6 ml) of high-titre viral vector at 1 × 108 transduction unit (TU)/ml (from about 3000 ml of supernatant) in one round of concentration. Electron microscopic analysis showed that PLL/viral vector formed complexes, which probably facilitated easy precipitation at low-speed concentration (10000 g), a speed which does not usually precipitate viral particles efficiently. Transfection of several cell lines in vitro and transduction in vivo in the liver with the lentivector/PLL complexes demonstrated efficient gene transfer without any significant signs of toxicity. These results suggest that the new method provides a convenient means for harvesting large volumes of high-titre lentivectors, facilitate gene therapy experiments in large animal or human gene therapy trials, in which large amounts of lentiviral vectors are a prerequisite.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Verma IM, Somia N . Gene therapy: promises, problems and prospects Nature 1997 389: 239–242
Amado RG, Chen IS . Lentiviral vectors – the promise of gene therapy within reach? Science 1999 285: 674–676
Kim VN, Mitrophanous K, Kingsman SM, Kingman AJ . Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1 J Virol 1998 72: 811–816
Naldini L et al. In vivo gene delivery and stable transduction of non-dividing cells by a lentiviral vector Science 1996 272: 263–267
Naldini L, Blömer U, Gage FH, Verma IM . Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector Proc Natl Acad Sci USA 1996 93: 11382–11388
Miyoshi H et al. Transduction of human CD 34+ cells that mediated long-term engraftment of NOD/SCID mice by HIV vectors Science 1999 283: 682–686
Miyoshi H, Takahashi M, Gage FH, Verma IM . Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector Proc Natl Acad Sci USA 1997 94: 10319–10323
Kafri T et al. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors Nat Genet 1997 17: 314–317
Burns JC et al. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmamalian cells Proc Natl Acad Sci USA 1993 90: 8033–8037
Uchida N et al. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic cells Proc Natl Acad Sci USA 1998 95: 11939–11944
Case SS et al. Stable transduction of quiescent CD34(+) CD38(−) human hematopoietic cells by HIV-1-based lentiviral vectors Proc Natl Acad Sci USA 1999 96: 2988–2993
Poeschla EM, Wong-Staal F, Looney DJ . Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors Nat Med 1998 4: 354–357
Olsen JC . Gene transfer vectors derived from equine infectious anemia virus Gene Therapy 1998 5: 1481–1487
Mselli-Lakhal L et al. Defective RNA packaging is responsible for low transduction efficiency of CAEV-based vectors Arch Virol 1998 143: 681–695
Metharom P et al. Novel bovine lentiviral vectors based on Jembrana disease virus J Gene Med 2000 2: 176–185
Berkowitz RD, Ilves H, Plavec I, Veres G . Gene transfer systems derived from visna virus: analysis of virus production and infectivity Virology 2001 279: 116–129
Paul RW et al. Increased viral titer through concentration of viral harvests from retroviral packaging lines Hum Gene Ther 1993 4: 609–615
Miller DL, Melkle PJ, Anson DS . A rapid and efficient method for concentration of small volume of retroviral supernatant Nucleic Acids Res 1996 24: 1577–1676
Bowles NE et al. A simple and efficient method for the concentration and purification of recombinant retrovirus for increased hepatocyte transduction Hum Gene Ther 1996 7: 1735–1742
Liu ML, Winther BL, Kay MA . Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)–Moloney murine leukemia virus-derived retrovirus vectors: comparison of VSV-G and amphotropic vectors for hepatic gene transfer J Virol 1996 70: 2497–2502
Hugues JP, Shen WC . Conjugation of methotrexate to poly (L-lysine) increases drug transport and overcomes drug resistance in cultured cells Proc Natl Acad Sci USA 1978 75: 3867–3870
Kichler A, Zauner W, Ogris M, Wagner E . Influence of the DNA complexation medium on the transfection efficiency of lipospermine/DNA particles Gene Therapy 1998 5: 855–860
Fasbender A et al. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vivo and in vitro J Biol Chem 1997 272: 6479–6489
Mochizuki H et al. High-titre human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells J Virol 1998 72: 8873–8883
Hodgson CP, Solaiman F . Virosomes: cationic liposomes enhance retroviral transduction Nat Biotech 1996 14: 339–342
Porter CD et al. Cationic liposomes enhance the rate of transduction by a recombinant retroviral vector in vitro and in vivo J Virol 1998 72: 4832–4840
Abe A, Chen S-T, Miyanohara A, Friedmann T . In vitro cell-free conversion of non-infectious Moloney retrovirus particles to an infectious form by the addition of the vesicular stomatitis virus surrogate envelope G protein J Virol 1998 72: 6356–6361
Sharma S, Miyanohara M, Friedmann T . Separable mechanisms of attachment and cell uptake during retrovirus infection J Virol 2000 74: 10790–10795
Acknowledgements
The authors wish to thank Ms P Metharom for assistance with the P24 assay; Dr J Mackenzie for materials and helpful advice on the gold immuno-labelling procedure; Mr C Winterford for assistance with the EM analysis, and Mr H Julie for analysis of fluorescence result. BZ is a Royal Children's Hospital Foundation/Chinese Club PhD scholar. This work was partly supported by a project grant to MQW from the National Heart Foundation and the Queensland Cancer Fund, Brisbane, Australia.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, B., Xia, H., Cleghorn, G. et al. A highly efficient and consistent method for harvesting large volumes of high-titre lentiviral vectors. Gene Ther 8, 1745–1751 (2001). https://doi.org/10.1038/sj.gt.3301587
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301587
Keywords
This article is cited by
-
Production of HIV-1-based virus-like particles for vaccination: achievements and limits
Applied Microbiology and Biotechnology (2019)
-
A new chemical complex can rapidly concentrate lentivirus and significantly enhance gene transduction
Cytotechnology (2018)
-
An HNF1α-regulated feedback circuit modulates hepatic fibrogenesis via the crosstalk between hepatocytes and hepatic stellate cells
Cell Research (2015)
-
Production, purification and titration of a lentivirus-based vector for gene delivery purposes
Cytotechnology (2014)
-
Simplified production and concentration of lentiviral vectors to achieve high transduction in primary human T cells
BMC Biotechnology (2013)