Abstract
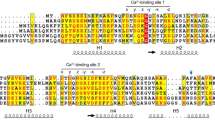
Many of the effects of calcium ions in eukaryotic cells are mediated by calcium-binding regulatory proteins such as calmodulin, in which each calcium-binding site has a distinctive helix–loop–helix conformation termed the EF hand1,2. Protein S from the spore coat of the Gram-negative bacterium Myxococcus xanthus has been shown to resemble calmodulin in its internally-duplicated structure and ability to bind calcium3. However, it has a β-sheet secondary structure4,5 rather than the helix–loop–helix arrangement of the eukaryotic proteins. We have determined the complete amino-acid sequence of a calcium-binding protein6 from the Gram-positive bacterium "Streptomyces erythraeus" by cloning and sequencing the corresponding gene. It contains four EF-hand motifs bearing remarkable sequence similarity to the calcium-binding sites in calmodulin. This implies that the EF-hand super-family may have evolved from ancient proteins present in prokaryotes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kretsinger, R. H. & Nockolds, C. E. J. biol. Chem. 248, 3313–3326 (1973).
Kretsinger, R. H. CRC crit. Rev. Biochem. 8, 119–174 (1980).
Inouye, S., Franceschini, T. & Inouye, M. Proc. natn. Acad. Sci. U.S.A. 80, 6829–6833 (1983).
Inouye, S., Harada, W., Zusman, D. & Inouye, M. J. Bact. 148, 678–683 (1981).
Wistow, G., Summers, L. & Blundell, T. L. Nature 315, 771–773 (1985).
Leadlay, P. F., Roberts, G. & Walker, J. E. FEBS Lett. 178, 157–160 (1984).
Cheung, W. Y. in Calcium and Cell Function Vol. 1 (ed. Cheung, W. Y.) 1–12 (Academic, New York, 1980).
Klee, C. B. & Vanaman, T. C. Adv. Protein Chem. 35, 213–321 (1982).
Van Eldik, L. J. & Watterson, D. M. in Calcium and Cell Physiology (ed. Marme, D.) 105–126 (Springer, Berlin, 1985).
Iwasa, Y., Yonemitsu, K., Matsui, K., Kukunaga, K. & Miyamoto, E. Biochem. biophys. Res. Commun. 98, 656–660 (1981).
Fry, L., Villa, I. J., Kuehn, G. D. & Hageman, J. H. Biochem. biophys. Res. Commun. 134, 212–217 (1986).
Sasagawa, T. et al. Biochemistry 21, 2565–2569 (1982).
Staden, R. Nucleic Acids Res. 10, 2951–2961 (1982).
Garnier, J., Osguthorpe, D. J. & Robson, B. J. molec. Biol. 120, 97–120 (1978).
Babu, Y. S. et al. Nature 315, 37–41 (1985).
Charbonneau, H. et al. Biochemistry 24, 6762–6771 (1985).
Ohno, S. et al. Nature 312, 566–570 (1984).
Parker, P. J. et al. Science 233, 853–859 (1986).
Davis, T. N., Urdea, M. S., Masiarz, F. R. & Thorner, J. Cell 47, 423–431 (1986).
Baum, P., Furlong, C. & Byers, B. Proc. natn. Acad. Sci. U.S.A 83, 5512–5516 (1986).
Salas, J. A., Guijarro, J. A. & Hardisson, C. J. Bact. 155, 1316–1323 (1983).
Eaton, D. & Ensign, J. C. J. Bact. 143, 377–382 (1980).
Goodman, M., Pechere, J.-F., Haiech, J. & Demaille, J. G. J. molec. Evol. 13, 331–352 (1979).
Carlson, T. A. & Chelon, B. K. Nature 322, 568–570 (1986).
Yates, L. & Vanaman, T. C. Fedn Proc. 40, 1738 (1981).
Labeda, D. P. Int. J. system. Bact. 37, 19–22 (1987).
Sanger, F., Nicklen, S. & Coulson, A. R. Proc. natn. Acad. Sci. U.S.A. 74, 5463–5467 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Swan, D., Hale, R., Dhillon, N. et al. A bacterial calcium-binding protein homologous to calmodulin. Nature 329, 84–85 (1987). https://doi.org/10.1038/329084a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/329084a0
This article is cited by
-
Immobilization of azide-functionalized proteins to micro- and nanoparticles directly from cell lysate
Microchimica Acta (2024)
-
Calcium Involved Directional Organization of Polymer Chains in Polyester Nanogranules in Bacterial Cells
Scientific Reports (2019)
-
Non-equilibrium dynamics of a nascent polypeptide during translation suppress its misfolding
Nature Communications (2019)
-
Structural basis for prokaryotic calciummediated regulation by a Streptomyces coelicolor calcium binding protein
Protein & Cell (2010)
-
Analysis of Acropora muricata Calmodulin (CaM) Indicates That Scleractinian Corals Possess the Ancestral Exon/Intron Organization of the Eumetazoan CaM Gene
Journal of Molecular Evolution (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.