Abstract
Study design:
Retrospective investigation using urodynamic studies and medical records.
Objective:
To evaluate the safety of sensation-dependent bladder emptying in complete spinal cord injury (SCI) patients, based on the preservation of the desire to void.
Setting:
Spinal Cord Injury Unit, Yonsei Rehabilitation Hospital, Seoul, Korea.
Methods:
This study was performed retrospectively on 79 complete SCI patients with lesions above T11, who had preserved the desire to void during conventional urodynamic studies. Patients were classified according to detrusor compliance and maximal bladder capacity. The clinical and urodynamic characteristics of each group were analyzed.
Results:
Forty-five (57.0%) patients were classified as group A and 34 (43.0%) patients were classified as group B. There were no significant differences in clinical features, such as voiding methods and the presence of autonomic dysreflexia between the two groups. Compared with group B, there were significantly more areflexic neurogenic bladder cases in group A (P<0.05). There were significantly higher maximal detrusor pressures in group B (P<0.05). There were significantly more cases with the preservation of the strong desire to void in group B (P<0.05).
Conclusion:
Not all patients with discomplete SCIs accepted the use of sensation-dependent bladder emptying. The safe use of sensation-dependent bladder emptying will be determined based on the results of urodynamic studies.
Similar content being viewed by others
Introduction
The neurogenic bladder in spinal cord injury (SCI) patients is an important part of rehabilitation, since it can cause significant morbidity and reduce the quality of life.1 Since Guttmann and Whitteridge2 reported their method using intermittent catheterization (IC) in 1947, it has become widely used for the urological management of patients with a neurogenic bladder due to SCI.3 Accordingly, for the effective management of the neurogenic bladder, much time and effort is provided in IC instruction from the start of an SCI rehabilitation program. Over time, many patients with SCI change their methods of voiding voluntarily because their voiding methods are troublesome. In 1998, Lim et al.4 reported that only 2 (6.7%) of 30 patients with SCI maintained the same IC method for a year after leaving the hospital. Because the appropriate voiding volume for the IC method is 400–450 ml at a time, a patient with an SCI must maintain a timely fluid intake of 1800–2000 ml per day and must perform catheterization every 4–6 h.5, 6 With such a time-dependent regimen, physiological changes in urinary excretion related to fluid intake or environmental changes may lead to unnecessary catheterizations and early emptying attempts or to overdistension of the bladder.7, 8 These difficulties are universally applicable to spontaneous and passive voiding by straining for maintenance of acceptable detrusor pressures and bladder volumes.9
Adequate sensory input is required for conscious bladder control and to store and expel urine at a convenient time and place. The hypogastric nerve (T11-L2) carries the most proximal spinal level.10 Although theoretically, a patient with a complete SCI above T11 is not able to transfer the desire to void from the bladder to the brain, some patients often express a weak desire to void or a different desire to void compared with their desire to void before their SCIs. In 1991, Wyndaele11 reported the existence of an afferent nerve pathway between the lower urinary tract and the cerebral cortex in 15 of 42 patients with complete SCIs. In 2006, we reported that there were 37 (31.6%) of 117 complete SCI patients with lesions above T11 with preservation of the desire to void.12
However, the clinical application of bladder emptying based on the preserved desire to void in patients with complete SCIs has accompanying problems, because there is controversy whether all of these patients have well-controlled neurogenic bladders. In patients with SCIs, the goal of management of neurogenic bladders is the preservation of renal function and maintenance of urinary continence for better social integration.13, 14 The low-compliance bladder receives special attention clinically because it causes deterioration of renal function as well as the development of hydronephrosis.15, 16 It is possible that there are many low-compliance neurogenic bladders in complete SCI patients with the preserved desire to void, because the preserved desire to void in complete SCIs will be a vague response to strong stimuli. Therefore, research should first consider how many patients with the preserved desire to void in complete SCIs have well-controlled neurogenic bladders.
The purpose of this study was to evaluate how many patients could use safely the sensation-dependent bladder emptying with preservation of the desire to void in above the T11 level in complete SCI patients, using retrospective analysis of urodynamic studies and medical chart reviews in these patients.
Patients and methods
Patients
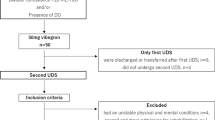
A total of 79 patients with complete lesions above T11, who preserved the desire to void during conventional urodynamic studies, were included in this study. Those who had a previous urologic disease, underwent urologic operations or had an associated peripheral neuropathy, such as diabetic neuropathy, were excluded. All subjects underwent electrophysiologic studies; including nerve conduction studies, electromyographies, pudendal nerve somatosensory evoked potential studies, and bulbocavernous reflex studies to confirm the presence of a complete SCI and to exclude peripheral neuropathies and sacral lesions. Informed consent was obtained from each patient and the Medical Ethical Committee at our hospital approved this study.
They were 56 male and 23 female patients. The mean age was 36.4 years (range: 19–74 years) and the mean time from injury was 23.2 months (range: 0.8–282.7 months). There were 57 tetraplegia and 75 traumatic SCI patients. The mean American Spinal Injury Association (ASIA) sensory score was 61.4 points (range: 13–136 points) on the basis of a total of 224 points (Table 1).13
Methods
Analysis of general characteristics
Patient data were obtained retrospectively using chart reviews. Age, sex, duration of injury, level of injury, cause of injury and ASIA sensory score were evaluated.
Analysis of urodynamic parameters
A urodynamic study was performed on each patient with a Duet urodynamic machine (Dantec, Denmark). This study was performed by an infusion of normal saline (20–30°C) at a rate of 30 ml/min through a double-lumen catheter, and a pressure sensor device at the rectum measured the abdominal pressure. When there was an involuntary leakage of normal saline up to 450–500 ml, an infusion was discontinued and the maximal bladder capacity was calculated as the volume at an involuntary leakage. When there was no involuntary leakage of neurogenic bladder up to 450–500 ml, an infusion was discontinued and the maximal bladder capacity was calculated as 450–500 ml.17 The bladder capacity and detrusor pressure at the first desire to void and the strong desire to void were evaluated prospectively during the filling phase. The maximal bladder capacity, maximal detrusor pressure, compliance, reflex volume and involuntary detrusor contraction were also evaluated. We calculated compliance by measuring the volume of the bladder until an abrupt rise in the detrusor pressure during the filling phase, and then dividing this volume by the detrusor pressure. If there was no involuntary reflex of neurogenic bladder, the compliance was calculated with the same method by using the volume of the bladder and the detrusor pressure when an infusion was discontinued.
We classified the neurogenic bladders into areflexic neurogenic bladders and neurogenic overactive bladders on the basis of the result of the urodynamic studies independent from the use of medication. For example, we classified a neurogenic bladder as areflexic if a patient, with the use of a medication, did not demonstrate an involuntary detrusor contraction during a conventional urodynamic study. We defined an overactive neurogenic bladder as an involuntary detrusor contraction with an increase of more than 15 cm H2O in detrusor pressure.18
Analysis of clinical parameters
Using chart reviews, we evaluated the voiding methods during the urodynamic studies, the existence of autonomic dysreflexia and central pain. We defined autonomic dysreflexia as more than one attack during 1 month and central pain as pain that necessitated medication use. We also evaluated the use of medications that influenced the neurogenic bladder and the existence of urinary tract infections. Medications that influenced the neurogenic bladder were defined as more than 1 week's use at the time of the urodynamic studies of: α-receptor blockers (doxazosin, tamsulosin and terazosin), anticholinergics (oxybutynin, propiverine, tolterodine and oxybutynin instillation). If a urine culture revealed more than 105 CFU/ml within 2 weeks of a urodynamic study and no antibiotics were prescribed, we defined that as the existence of a urinary tract infection.
Grouping
We classified group A as those with a maximal bladder capacity of more than 400 ml and a compliance greater than 15 ml/cm H2O. All others were classified in group B.1, 16 We performed comparisons of general characteristics and urodynamic and clinical parameters between the two groups.
Statistical analysis
For statistical analysis, SPSS 12.0 Windows version, the χ2 test, and the independent t-test were used. Statistical significance was defined as P<0.05.
Results
Among the 79 patients, 45 (57. 0%) patients were classified as group A and 34 (43.0%) patients were classified as group B (Table 2).
Comparison of general characteristics between the two groups
There was no statistically significant difference between the two groups with respect to age, sex, duration of injury, level of injury, cause of injury or ASIA sensory score (Table 3).
Comparison of urodynamic parameters between the two groups
The mean maximal bladder capacity, the mean maximal detrusor pressure and the mean compliance of group A were statistically significantly different from those of group B (P<0.05; Table 4).
All patients preserved the first desire to void.
The mean bladder capacity at the first desire to void (281.4 ml) in group A was statistically significantly greater than that (214.6 ml) in group B (P<0.05). The mean detrusor pressure at the first desire to void (15.8 cm H2O) in group A was statistically significantly lower than that (30.5 cm H2O) in group B (P<0.05; Table 4).
The number of cases with the preservation of the strong desire to void (nine cases, 20%) in group A was statistically significantly less than that (15 cases, 44.1%) in group B (P<0.05). The mean bladder capacity at the strong desire to void (397.6 ml) in group A was statistically significantly greater than that (298.0 ml) in group B (P<0.05). The mean detrusor pressure at the strong desire to void (24.6 cm H2O) in group A was statistically significantly lower than that (51.5 cm H2O) in group B (P<0.05; Table 5).
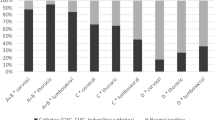
In group A, there were 37 cases with the bladder capacity and the detrusor pressure at the preserved desire to void (the first desire to void or the strong desire to void) were, respectively, greater than 150 ml and less than 40 cm H2O. In group B, there were 17 cases with the above conditions (Table 6).
In group A, there were 26 cases with areflexic neurogenic bladders. In group B, there were two cases with areflexic neurogenic bladders. The number of areflexic neurogenic bladders was statistically significantly greater in group A than in group B (P<0.05).
Comparison of clinical parameters between the two groups
There were no significant differences in the voiding methods, the existence of autonomic dysreflexia, central pain, urinary tract infections and the use of medications (Table 7).
Discussion
For investigation of sensory preservation in complete SCIs, the term discomplete SCI was first introduced by Dimitrijevic19 to describe a clinically complete SCI with neurophysiological evidence of residual brain influence on spinal cord function below the lesion. Finnerup et al.20 reported that painful or repetitive stimuli below the lesion elicited vague localized sensations in 19 of the 24 patients with complete SCIs and used the term of ‘sensory discomplete’. They suggested that the mechanisms of discomplete SCI were such that spinal sympathetic reflex activation was likely to be responsible for the diffuse chills and sweating in the face described by some patients; and muscle groups innervated from segments above the spinal cord injury may register proprioceptive impulses arising from muscle spasms. But they also reported that prolonged painful or repetitive stimuli may pass through axons in the injured cord when a threshold is exceeded or through a summation phenomenon, because in 12 patients, painful or repetitive pinpricks elicited more localized sensations, although not specific for the modality used and not characterized as pain by the patients.
The present study suggests that there were 79 patients with complete SCIs, who had the preserved desire to void, and there were discomplete SCIs that had some neurophysiological communication between the segments above and below the spinal cord lesions in these clinically complete SCIs.
During the investigation of the normal pattern of perception of bladder filling, the bladder volume and detrusor pressure at the first desire to void and those at the strong desire to void were, respectively, 326.1±147.8 ml and 9.3±8.8 cm H2O, and 562.7±133.9 ml and 12.4±10.8 cm H2O in the case of men; and respectively, 211.3±92.8 ml and 6.0±4.2 cm H2O, and 456.1±143.3 ml and 7.3±5.0 cm H2O in the case of women.21 In the normal bladder, the changes of bladder hydrostatic pressure induce the depolarization of the bladder mucosa. The electrical potential difference produces a release of ATP that activates the ATP receptor, P2X3, in small diameter sensory neurons. These processes activate the afferent nerves of the A-δ fibers.22, 23 Storage and periodic release of urine depend on the coordinated activity of the lower urinary tract. This coordination is mediated by sympathetic, parasympathetic and somatic reflex pathways in the brain and spinal cord.24 The brain regions activated by bladder distention are the periaqueductal gray, rostral pons, anterior cingulate gyrus, anterior insula, putamen, thalamus and cerebellum; whereas, those activated by intravesical ice water instillation are mainly the frontal and parietal cortex, amygdala – hippocampus area, and crus cerebri ventral border.25
In patients with SCIs, small unmyelinated C-fibers, which detect mainly noxious signals and initiate nociceptive sensations, become relevant after disconnection of the bladder from a higher center in addition to small myelinated A-δ fibers, which detect bladder fullness or wall tension.25 However, the roles of each A-δ fibers and C-fibers that ice water activated have not been determined definitively in neurogenic bladders of patients with SCIs. Further study will be necessary in an effort to better understand the problem on the basis of the fact that the brain regions activated by bladder distention are different from those activated by intravesical ice water instillation.
During the investigation of the neurogenic bladder in discomplete SCIs, Wyndaele11 reported that 15 of the 42 patients with a lesion diagnosed clinically as complete, perceived bladder filling, indicated the existence of an afferent nerve pathway between the lower urinary tract and the cerebral cortex. Ersoz and Akyuz26 reported that 6 of the 18 patients with complete SCIs above T11 had preserved the bladder-filling sensations, and sensation-dependent bladder emptying was possible in these patients because the bladder capacity and the detrusor pressure at the preserved bladder-filling sensation were, respectively, greater than 150 ml and less than 40 cm H2O. When these data were analyzed, based on the article by Ersoz and Akyuz,26 54 (68.4%) patients in the present study were in the group in which the sensation-dependent bladder emptying seemed possible. However, 17 of these 54 patients demonstrated that the compliances were less than 15 ml/cm H2O or that the maximal bladder capacities were less than 400 ml during continuous urodynamic studies. Only 37 (46.8%) patients in this study were able to empty their bladders using the preserved desire to void and these were in group A. In a broad sense, 45 patients (57.0%) in this study had the well-controlled neurogenic bladders and were able to empty their bladders safely. All these 45 patients belonged to group A in this study. In addition, because there was no difference in the general characteristics and the clinical parameters, we could not classify patients with discomplete SCIs in groups A and B if these were not made during the urodynamic studies.
In this study, the cases with preservation of the strong desire to void were 13.8% in group A compared with 44.1% in group B. This result could be due to our urodynamic protocol that limited the maximal bladder capacity to 450–500 ml. If a continuous infusion of normal saline over 450–500 ml were performed in group A, more patients could have expressed the strong desire to void. But these results may be used as a clinical clue for sensation-dependent bladder emptying in patients with discomplete SCIs. Although a urodynamic study is performed in an artificial environment, the bladder of a patient with a discomplete SCI will be a low-compliance bladder if the patient shows frequent strong desires to void in the clinical environment. This observation, however, needs further studies because we did not evaluate which, if any, connection existed between the strong desire to void during urodynamic studies and in the clinical environment since this study used retrospective analysis.
There was no significant difference between groups A and B in the use of medications that influenced the neurogenic bladders. This result may mean that the use of medications that influenced the neurogenic bladders did not have any effect on the preserved desire to void. However, it is not known whether the preserved desire to void may disappear after the use of medications in group B. If the preserved desire to void may still remain after the control of the low compliance by means of the use of medication, it is possible that the sensation-dependent bladder emptying is performed in the low-compliance bladder. However, it is not known whether the preserved desire to void may disappear after the use of medications. Further studies will be required to determine this.
The low-compliance neurogenic bladder is clinically receiving special attention because it causes deterioration of renal function and also causes hydronephrosis.1 This study demonstrated that not all patients with discomplete SCIs accepted the use of sensation-dependent bladder emptying. However, the use of sensation-dependent bladder emptying will be determined based on the results of urodynamic studies if patients with discomplete SCIs express the desire to void.
According to our data, sensation-dependent bladder emptying was acceptable in 45 patients who expressed the desire to void and had the well-controlled neurogenic bladders. However, there was no evidence of the clinical use of sensation-dependent bladder emptying in these patients, since this study used retrospective analysis. This is a limitation to our study. Therefore, further studies will be required to determine whether sensation-dependent bladder emptying in patients with discomplete SCIs will affect the management of neurogenic bladders and the quality of life.
Conclusion
To summarize, the desire to void was preserved in 79 patients with clinically complete SCIs above T11. There were 45 (57.0%) of 79 patients with well-controlled bladders. There were no significant differences in general characteristics and clinical features. Those with preserved desires to void were not necessarily accurate in the determination of bladder emptying time with discomplete SCIs. Urodynamic studies must be performed first before attempting a trial of sensation-dependent bladder emptying.
References
Shin JC, Park CI, Kim HJ, Lee IY . Significance of low compliance bladder in cauda equina injury. Spinal Cord 2002; 40: 650–655.
Guttmann L, Whitteridge D . Effects of bladder distension on autonomic mechanism after spinal cord injuries. Brain 1947; 70: 361–404.
Maynard FM . Long-term management of neurogenic bladder: intermittent catheterization. Phys Med Rehabil Clin N Am 1993; 4: 299–308.
Lim JK, Choi YS, Park CB, Ryu SY, Yu HJ, Cho KH et al. Postdischarge change of neurogenic bladder management methods in spinal cord injured patients. J Korean Acad Rehabil Med 1998; 24: 1044–1048.
Giannantoni A, Scivoletto G, Di Stasi SM, Silecchia A, Finazzi-Agro E, Micali I et al. Clean intermittent catheterization and prevention of renal disease in spinal cord injury patients. Spinal Cord 1998; 36: 29–32.
Karlsson AK . Autonomic dysreflexia. Spinal Cord 1999; 37: 383–391.
Anton HA, Chambers K, Clifton J, Tasaka J . Clinical utility of a portable ultrasound device in intermittent catheterization. Arch Phys Med Rehabil 1998; 79: 172–175.
De Ridder D, Van Poppel H, Baert L, Binard J . From time dependent intermittent self-catheterisation in multiple sclerosis using the PCI 5000 Bladdermanager. Spinal Cord 1997; 35: 613–616.
Downey JA, Myers SJ, Gonzalez EG, Lieberman JS . The Physiological Basis of Rehabilitation Medicine, 2nd edn. Butterworth-Heinemann: Boston, 1994, pp 504–506.
De Groat WC . Anatomy and physiology of the lower urinary tract. Urol Clin N Am 1993; 20: 383–401.
Wyndaele JJ . Investigation of the afferent nerves of the lower urinary tract in patients with ‘complete’ and ‘incomplete’ spinal cord injury. Paraplegia 1991; 29: 490–494.
Shin JC, Kang SW, Chang WH, Jung TH, Yoo JH, Mah SY et al. Desire to void in patients with complete spinal cord injury. J Korean Acad Rehabil Med 2006; 30: 340–345.
Frost FS . Spinal cord injury medicine. In: Braddom RL, Buschbacher RM, Dumitru D, Johnson EW, Matthews DJ, Sinaki M, (eds), Physical Medicine and Rehabilitation, 2nd edn, Saunders: Philadelphia, 2000, pp 1231–1282.
McGuire EJ, Cespedes RD, O'Connell HE . Leak-point pressures. Urol Clin N Am 1996; 23: 253–262.
Hackler RH, Hall MK, Zampieri TA . Bladder hypocompliance in the spinal cord injury population. J Urol 1989; 141: 1390–1393.
Shin JC, Kim YW, Park CI, Kang SW, Yang SC . Effect of the intravesical resiniferatoxin instillation evaluated by the ice provocative urodynamic study. Spinal Cord 2006; 44: 309–314.
Park CI . Urodynamic studies in neurogenic bladder. J Korean Acad Rehabil Med 1991; 15: 139–142.
Stohrer M, Goepel M, Kondo A, Kramer G, Madersbacher H, Millard R et al. The standardization of terminology in neurogenic lower urinary tract dysfunction with suggestions for diagnostic procedures. Neurourol Urodyn 1999; 18: 139–158.
Dimitrijevic MR . Neurophysiology in spinal cord injury. Paraplegia 1987; 25: 205–208.
Finnerup NB, Gyldensted C, Fuglsang-Frederiksen A, Bach FW, Jensen TS . Sensory perception in complete spinal cord injury. Acta Neurol Scand 2004; 109: 194–199.
Wyndaele JJ . The normal pattern of perception of bladder filling during cystometry studied in 38 young healthy volunteers. J Urol 1998; 160: 479–481.
Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000; 407: 1011–1015.
Ferguson DR, Kennedy I, Burton TJ . ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol 1997; 505: 503–511.
Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T et al. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J Urol 2002; 168: 2035–2039.
Kaplan SA, Blaivas JG, Breuer A . Urogenital physiology. In: Downey JA, Myers SJ, Gonzalez EG, Lieberman JS (eds), The Physiological Basis of Rehabilitation Medicine, 2nd edn. Butterworth-Heinemann: Boston, 1994, pp 501–517.
Ersoz M, Akyuz M . Bladder-filling sensation in patients with spinal cord injury and the potential for sensation-dependent bladder emptying. Spinal Cord 2004; 42: 110–116.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, J., Chang, W., Jung, T. et al. The determination of sensation-dependent bladder emptying time in patients with complete spinal cord injury above T11. Spinal Cord 46, 210–215 (2008). https://doi.org/10.1038/sj.sc.3102102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3102102
Keywords
This article is cited by
-
Combining different evaluations of sensation to assess the afferent innervation of the lower urinary tract after SCI
Spinal Cord (2021)
-
Urodynamic findings, bladder emptying methods and therapeutic approaches in patients with upper lumbar and lower lumbar–sacral spinal cord injury
Neurological Sciences (2015)
-
Protective effect of preserved bladder-filling sensation on upper urinary tract in patients with spinal cord injury
Neurological Sciences (2014)
-
The assessment of bladder and urethral function in spinal cord injury patients
Journal of Huazhong University of Science and Technology [Medical Sciences] (2009)