Abstract
Study design:
Case report.
Objective:
To evaluate the functional recovery of chronic complete idiopathic transverse myelitis (ITM) after administration of acidic fibroblast growth factor (aFGF).
Methods:
A 28-year-old woman presented with a 4-year history of spastic paralysis, sensory level at T10, urinary retention and constipation due to ITM. In all, 20 μg aFGF bolus injection was applied via intradural lumbar puncture, which was repeated every 5 months for 15 months.
Results:
At 3 weeks after first injection, the patient experienced vague sensation at approximately T12–L1 dermatomes. At 2 months after the second injection, muscle activities and gait pattern were recorded in bilateral gluteus and hip abductors as she ambulated with long leg brace and axillary crutches. Increased walking speeds, reduced pelvic tilting and reduced compensatory trunk rotation during the swing phase were also demonstrated as compared to the initial gait analysis. At 18 months after injection, motor evoked potentials were obtained in hip abductors of both legs.
Conclusions:
aFGF may increase the efficacy of spinal reactivation/regeneration and is a potential remedy for chronic transverse myelitis.
Similar content being viewed by others
Introduction
Transverse myelitis (TM) is a rare neuroimmunologic disorder, characterized by focal spinal cord inflammation and the resulting neural injuries. The causes of TM are uncertain and may be idiopathic; therefore, the elimination of potential treatable sources is vital for medical treatment. In previous studies, either partial or complete functional recovery has been achieved in disease-associated acute transverse myelitis (ATM) treated with high-dose corticosteroid, hydroxychloroquine, plasmapheresis and pulse cyclophosphamide, whereas no clearly established medical treatment currently exists for idiopathic transverse myelitis (ITM).1
The pathophysiology and course of ATM has been studied extensively. An intraparenchymal or perivascular cellular influx into the spinal cord results in the breakdown of the blood–brain-barrier and variable demyelination and neuronal injury.2 The formulation of strategies to enhance axon and oligodendrocyte regeneration, as well as peripheral neural regrowth, are therefore major research goals. In vitro effects of fibroblast growth factors include promotion of various central and peripheral neuronal populations survival and mitogenesis in a variety of non-neuronal cell types.3 Consequently, the possibility of growth factor use in the treatment of diseased-associated TM has been considered and, recently, acidic fibroblast growth factor (aFGF) treatment has been shown to prevent motoneuron loss,4 improve corticospinal tract regeneration,5 and contribute to angiogenesis in vitro.6 Some studies suggest that the use of corticosteroids in ITM patients at the acute stage results in a modest potential increase in functional recovery; however, currently no bold therapies for chronic stage ITM are available.7 We present a unique case involving a chronic complete paraplegic patient of ITM treated with aFGF.
Case report
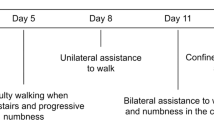
A 28-year-old female patient arrived at our hospital seeking medical attention in January 2001. A 4-year history of spastic paralysis over bilateral lower limbs with sensory level at T10, urinary retention and constipation were presented.
In March 1997 at the age of 24, she exhibited progressive loss of strength in both legs with sensory level at T5, impaired sphincter function, and reached her maximum deficits within 4 weeks. The MRI revealed multiple areas of abnormally increased signal intensity involving whole thoracic cord on both T2W1 and gradient echo. A diagnosis of TM with uncertain causes was made when cytochemical study of cerebrospinal fluid, serologic tests, spinal arteriography, brain stem evoked potential were noncontributory to the diagnosis. In spite of a series of aggressive multidisciplinary rehabilitation programs for 1 year, due to spastic complete paralysis in both legs and weakness of trunk, mobility by wheelchair was necessary.
On her March 12, 2001 admission she presented with absent pin-prick, light touch, pain and temperature, vibration and proprioception sensations below bilateral T10 levels. Sacral sensation was also absent. Urodynamic study revealed a hyperreflexic bladder with poor compliance and low intravesical pressure. She was then treated with a first dose of 20 μg acidic FGF (R&D Systems Inc. 614 Mckinley Place N.E., Minneapolis, MN 55413, USA) added in fibrin glue bolus injections via lumbar puncture, and then every 5 months for a total of 15 months, according to the treatment regimen in our previous studies. The fibrin glue (BeriplastTMP, Behring, Germany) was prepared before use by mixing the fibrinogen (100 mg/ml) with aprotinin solution (200 KIU/ml) plus calcium chloride (8 mM). The final glue volume was about 3 ml. The process adhered to the Occupational Health and Safety Administration regulations, and received prior approval from the local institutional board and the National Organization for Human Research. At 3 weeks after first injection, she experienced vague pin-prick and light touch sensation at approximately T12–L1, while pain and temperature, vibration and proprioception sensations remained absent.
She also received a 30-min gait training on a treadmill at a speed of 1.5 mph with 30% of body weight support, three times a week for 6 weeks, from August 2001 to October 2001.
At 2 months after the second injection, she received a computerized high-resolution three-dimensional motion analysis for baseline study by Vicon 370 (Oxford Metrics, Oxford, UK), muscle activities recording, and heart rate monitoring to evaluate her motor function objectively. As she ambulated with long leg brace and axillary crutches, muscle activities were recorded in bilateral gluteus maximus, gluteus medius, and hip abductors by surface recording electrodes. As for motion analysis, 13 reflective markers were placed on the sacrum, bilateral anterior superior iliac spines, and lateral aspects of both the long leg braces including hip joints, mid-thighs, knee joints, mid-shanks, and bilateral lateral malleoluses, and the second metatarsal heads. Only hip joint kinematics was analyzed because wearing long leg braces controlled and restricted knee and ankle joint function. The patient did not receive motion analysis before the treatment since only wheelchair ambulation could be achieved at that time.
The second gait analysis was done 6 months later, 1 month after the third injection. As for the hip joint kinematics, left hip joint excursion ([maximal–minimal] flexion–extension angles) increased 12.44% and right hip joint excursion increased 1.56%. Gait speed improved 10.11%, cadence improved 20%, and 2-min maximum walking distance improved 13.33%; however, the stride length declined 6.15%. The Physiological Cost Index during the 5-min walking period improved 7.57%. The muscle activation pattern of bilateral gleuteus maximus and gleuteus medius during gait cycle revealed that these four muscles seem to act as hip flexors at the late swing phase to help foot clearance. However, bilateral adductors magnus, rectus femoris, lateral hamstring, and gastrocnemius did not show significant changes. Increased walking speeds, reduced pelvic tilting, and reduced compensatory trunk rotation during the swing phase were also demonstrated as compared to the initial gait analysis.
Since the aFGF injection and 6, 18 months thereafter, we made representative assessments of motor, sensory, and bladder impairment to determine whether intrathecal neurotrophin administration contributed to nerve regeneration and functional restoration. The electrophysiological investigations for each time point are summarized in Figure 1. Before intervention the motor evoked potentials (MEPs), somatosensory evoked potentials (SEPs) were not evident. The SEPs remained unobtainable throughout the course, but MEPs were elicited at L1/L2 paraspinal muscles and tensor fascia lata 1½ year after injection. The repeated urodynamic examination remained unchanged. The patient was found still to have a hyperreflexic bladder with poor compliance, regardless of the aFGF treatment.
Discussion
The present article describes the findings for a patient who presented with a history of complete spastic paralytic ITM for four years before visiting our clinic. Initial presentations included spinal shock and the rapid progression of back pain. A poor outcome was predicted by absent central conduction on evoked potential testing. It is hoped, however, that by intervening in the degeneration process the final outcome of TM may be experimentally manipulated. Several pioneering studies have laid the groundwork for these new prospects. Webster8 has demonstrated that the actions of growth factors might help reduce oligodendrocyte-myelin sheath injury in multiple sclerosis and promote myelin regeneration. Albrecht et al9 found ciliary neurotrophic factor to be an important cytokine in demyelinating disease as well as an upstream regulator of FGF-2 production in astrocytes during early remyelination. This suggests that FGF may be neuroprotective and stimulate neural growth and proliferation. The effectiveness of growth factors in realistic models has not yet been substantiated in clinical situations.
Unlike spinal cord injury, in which cords were contused and spinal axons were transected, most TM victims have residual axons crossing the implicated site. The vague sensation noted in T12–L1 of this patient at 3 weeks after first injection with aFGF is greater than the average rate of axonal growth observed in human nerves, suggesting that sensory nerve regeneration is not the contributor to early recovery; however, reactivation of some surviving viable sensory neurons might be an important factor. Presently, the origin, mechanism, or pathway for this event still remains unknown.
Neurotrophic factors in general have poor pharmacokinetic profiles and short in vivo half-life.10 Our previous study using [125I]-labeled GDNF suggested that fibrin glue is an effective substrate for keeping a trophic factor localized in situ for a finite period, protected from the circulation, surrounding aqueous humor or cerebrospinal fluid (CSF).11 Mixing aFGF with fibrin glue allows for slow release of the growth factor; however, their access to the central nervous system (CNS) is largely impeded by multiple clearance processes.12 Therefore, aside from the initial injection, we performed three sequential lumbar punctures every 5 months for a total of 15 months.
It took at least 1 year after aFGF injection for locomotor responses and magnetic motor response to return. This may demonstrate that the synthesis rate for new synapses and axonoplasmic transport determines the CNS synapse growth rate and the duration of motor recovery. During the 18 months following aFGF injection some of the patient's regenerated motor axons experienced a degree of remyelination and maturation. This partially accounts for the delay in latency and the modest amplitude of the patient's motor response.
Recent studies supported the concept of therapeutic treatment approach with supported treadmill ambulation training for spinal cord injury based on evidence that central pattern generators in the spinal cord produce specific, rhythmic movements of limb flexor and extensor muscle groups devoid of any supraspinal or sensory inputs.13 It is reported that this training approach improves speed of ambulation, improves endurance, and achieves as normal a gait as possible.14 Thus, although supported treadmill ambulation training alone will not enable people with severe spinal cord injury to walk, with respect to our case, its contribution to improve endurance and gait pattern cannot be excluded.
In this report, the patient presented some motor recovery during the 18 months after aFGF injections, but a full recovery was not achieved. The acute inflammatory process that induced neural injury in the spinal cord may have contributed to the loss of axotomized motor neurons, thus restricting a complete recovery.15 Alternatively, this might be explained by the brief half-life of aFGF.16 Most neurotrophic factors have poor pharmacokinetic profiles and short in vivo half-lives. In our case, fibrin glue was used to slow the release of growth factor and prolong its effect. Additionally, although delivery of neurotrophic factors into the CSF following intrathecal administration is not invasive and allows access to a much wider area of the CNS through CSF circulation pathways, their path to the CNS is blocked by multiple clearance activities. New methods of targeted drug delivery for neurotrophic factors will therefore be crucial in determining clinical efficacy in CNS conditions.
Recent studies revealed that aFGF facilitates nerve regeneration and increases the efficacy of sprouting in rats with spinal cord and cervical root transection.17, 18, 19 Our patient responded well to intrathecal application of aFGF. She had no side effects, and the results of the treatment could be maintained under a regular rehabilitation program. Based on these promising results, we believe that the use of aFGF to treat patients with TM from uncertain origin should be further evaluated.
Conclusion
Although this case was limited to a single patient, the study shows that locomotor activity improvement may be possible in chronic paralytic patients of ITM using intrathecal aFGF. A future trial including additional patients is necessary in order to determine whether FGF is the best growth factor to apply.
References
Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002; 59: 499–505.
Kerr DA, Ayetey H . Immunopathogenesis of acute transverse myelitis. Curr Opin Neurol 2002; 15: 339–347.
Stock A et al. Localization of acidic fibroblast growth factor in specific subcortical neuronal populations. J Neurosci 1992; 12: 4688–4700.
Guest JD et al. Influence of IN-1 antibody and acidic FGF-fibrin glue on the response of injured corticospinal tract axons to human Schwann cell grafts. J Neurosci Res 1997; 50: 888–905.
Cuevas P, Carceller F, Gimenez-Gallego G . Acidic fibroblast growth factor prevents death of spinal cord motoneurons in newborn rats after nerve section. Neurol Res 1995; 17: 396–399.
Javerzat S, Auguste P, Bikfalvi A . The role of fibroblast growth factors in vascular development. Trends Mol Med 2002; 8: 483–489.
Kelley CE, Mathews J, Noskin GA . Acute transverse myelitis in the emergency department: a case report and review of the literature. J Emerg Med 1991; 9: 417–420.
Webster HD . Growth factors and myelin regeneration in multiple sclerosis. Mult Scler 1997; 3: 113–120.
Albrecht PJ et al. Astrocytes produce CNTF during the remyelination phase of viral-induced spinal cord demyelination to stimulate FGF-2 production. Neurobiol Dis 2003; 13: 89–101.
Thorne RG, Frey II WH . Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet 2001; 40: 907–946.
Cheng H et al. Characterization of a fibrin glue-GDNF slow-release preparation. Cell Transplant 1998; 7: 52–61.
Cheng H, Cao Y, Olson L . Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 1996; 273: 510–513.
Basso DM . Neuroanatomical substrates of functional recovery after experimental spinal cord injury: implications of basic science research for human spinal cord injury. Phys Ther 2000; 80: 808–817.
McDonald JW, Sadowsky C . Spinal cord injury. Lancet 2002; 359: 417–425.
Li XK et al. Pharmacokinetic study of recombinant human acidic fibroblast growth factor in rabbits by skin external use. Yao Hsueh Hsueh Pao – Acta Pharmaceutica Sinica 2002; 37: 424–427.
Thorne RG, Frey II WH . Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet 2001; 40: 907–946.
Cheng H, Cao Y, Olson L . Spinal cord repair in adult paraplegic rats: partial restoration of hindlimb function. Science 1996; 273: 510–513.
Chuang TY et al. Forelimb muscle activity following nerve graft repair of ventral roots in the rat cervical spinal cord. Life Sci 2002; 71: 487–496.
Lee YS et al. aFGF promotes axonal growth in rat spinal cord organotypic slice co-cultures. J Neurotraum 2002; 19: 357–367.
Acknowledgements
The authors gratefully acknowledge the contribution of Professor Lars Olson at Department of Neuroscience, Karolinska Institute in Stockholm, Sweden, for his assistance in the laboratory work. This work was supported by Taipei Veterans General Hospital, Grant number: VGH 93-371.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lin, PH., Chuang, TY., Liao, KK. et al. Functional recovery of chronic complete idiopathic transverse myelitis after administration of neurotrophic factors. Spinal Cord 44, 254–257 (2006). https://doi.org/10.1038/sj.sc.3101809
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101809
Keywords
This article is cited by
-
Functional improvement in chronic human spinal cord injury: Four years after acidic fibroblast growth factor
Scientific Reports (2018)
-
The FGF family: biology, pathophysiology and therapy
Nature Reviews Drug Discovery (2009)