Abstract
Study design:
A prospective observational study.
Objective:
To evaluate bone mineral density (BMD) and biochemical markers of bone turnover in spinal cord-lesioned females in the early postmenopausal period.
Setting:
Clinic for Spinal Cord Injuries, H:S Rigshospitalet, Denmark.
Material:
In all, 18 early postmenopausal females with spinal cord lesions (SCL) of more than 2 years duration were recruited. In total, 11 completed the study.
Methods:
Using dual energy X-ray absorption, BMD of the lumbar spine, femoral neck, trochanter and proximal tibia was measured every 6 months for 30 months. Biochemical markers of bone turnover in blood and urine were collected at the same time points.
Results:
A significant increase in markers of bone formation in the blood was found and markers of bone resorption in urine tended to increase. BMD values changed insignificantly but for all regions decreased, except the lumbar spine.
Conclusion:
An accelerated bone turnover occurs in early postmenopausal SCL females. At the same time, we showed an insignificant decrease in BMD data from the lower extremity.
Similar content being viewed by others
Introduction
Bone loss in spinal cord-lesioned (SCL) persons is well known.1, 2, 3, 4, 5 There is a correlation between the decrease in bone mineral density (BMD) and fracture incidence.6, 7 The overall fracture rate is 2% per year in the Danish SCL population compared to 1% per year in healthy controls.8 In the same study, the relative risk of femur fractures in the SCL population was 23 times higher than for the controls.
Normal females lose bone mass in the leg at a rate of 4% per year early after menopause.9 On average, the bone loss is 20–25% over a 16-year period after menopause regardless of region.10 In SCL females, the trajectory of bone loss during and after menopause has not been studied.
Antiosteoporotic treatment is not routine in persons with SCL, but has been studied earlier and recently.11, 12, 13, 14
The aim of this study was to follow the trajectory of bone loss early after menopause in SCL women.
Materials and methods
Participants
In all, 84 females aged 38–59 years from the patient register of the Clinic for Spinal Cord Injuries were asked to participate in the study. In total, 51 of them agreed to participate and then received a screening questionnaire. A total of 18 subjects were included. Neurologically, the participants were characterized by the level of SCL and Frankel grade.15
They gave oral and written informed consent, and the study was approved by the Ethical Committee of Copenhagen Municipality no. 01-234/95.
The women were considered peri- or postmenopausal if they had experienced irregular or discontinued menstrual bleedings and/or classical climacteric symptoms (the latter symptom alone if hysterectomy had taken place). The plasma level of follicle-stimulating hormone was determined in subjects with climacteric symptoms alone. Two subjects (nos. 2 and 6, Table 1) were perimenopausal by study start and proved postmenopausal within 18 months later, when their menstruations completely ceased. Women were excluded if they were using estrogen or other medication affecting calcium homeostasis (eg steroids, bisphosphonates), had known metabolic disorders (except stable diabetes and hyperthyroidism), known liver or intestinal disease, malignancy, chronic alcoholism and a psychiatric disease affecting an informed consent or cooperation.
Methods
Standard blood tests (hemoglobulin, P-creatinine, P-Ca, PTH, TSH, alkaline phosphatases) were performed to screen for any disease affecting calcium homeostasis.
Dual energy X-ray absorptiometry (Norland XR 26 MK1) measured BMD of the lumbar spine, hip and tibia every 6 months for 2.5 years.
The spine, hip and tibia were measured in the anteroposterior projection, with the patient in a supine position with her heels in a standardized support device. The proximal tibia was scanned in the small animal program. Pixel size was 0.5 × 0.5 cm. Test–retest procedures for BMD measurements were carried out on three participants.
Blood and urine samples were collected on the day of examination for analysis of se-osteocalcin (precision 6.5%), se-alkaline phosphatases (precision 3%), urine-hydroxyproline (precision 13 μmol) and urine-calcium (precision 0.1 mM). Urine parameters were corrected for creatinine secretion (precision 2%).
Statistics
The coefficient of variation was calculated for the BMD measurements test–retest.
Repeated measures analysis of variance was performed on logarithmed data using PROC GLM in SAS. Owing to the small number of subjects, the testing was restricted to significance of linear contrasts. This is equivalent to calculating a linear fit of the response versus time and performing a one-sample t-test for the hypothesis that the estimated slopes have mean zero.
After stratifying according to time since injury and to Frankel grade, Mann–Whitney test was also performed.
The level of statistically significance was chosen at 0.05.
Results
Two persons dropped out because of continued use of estrogen, which after initial confirmation they were nevertheless not willing to discontinue. Three were not menopausal according to the blood test, and one was on steroid therapy (connective tissue disease), in spite of a negation of this in the questionnaire. One dropped out half way through the study because of recurrent airway infections.
The remaining 11 women completed all the sessions. For those who completed, the study age ranged from 43 to 58 years (median 52), time since menopause ranged from 0 to 7 years (median 4) and time since SCL ranged from 3 to 49 years (median 11). The patient characteristics are described in Table 1.
For the test–retest procedure, the coefficients of variation in BMD were all less than 3.3% (median 1.3%).
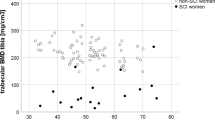
BMD of the femoral neck and trochanter and of the tibia decreased by 7.6, 5.7 and 2.7%, respectively (P-values 0.16, 0.14 and 0.14). BMD of the spine decreased by 0.7% (P-value 0.29).
There were no differences in BMD values between groups, neither after stratifying the subjects in two subgroups according to time since lesion (subjects 4 and 6–10 versus the remaining, see Table 1) nor after stratification by Frankel grade (seven subjects with Frankel grade D and four subjects with grades A–C, see Table 1).
Over the period of 30 months, osteocalcine and alkaline phosphatase values increased by 36 and 8%, respectively (P-values 0.01 and 0.05). Urine-calcium increased by 106% and urine-hydroxyproline by 194% (P-values 0.06 and 0.10). The results are presented in Tables 2 and 3.
Discussion
Our results showed a decline in BMD of the femoral neck, trochanter and tibia, which, however, did not reach significance. A plausible reason might be the small number of participants in the study. The bone loss in the femoral neck and trochanter is substantial and deserves some consideration. The loss amounted to 3% per year, and this seems to exceed what would be expected from immobilization alone in a chronic SCL population over a 2.5-year period.
In able-bodied females, there is an expected bone loss of 4% in the leg per year in early menopause9 and of 20–25% in average regardless of region over a 16-year period following menopause, and additionally it has been shown that the bone loss in the femoral neck is 1% per year 10 years after menopause.10
While acute immobilization in SCL patients results in a rapid decrease in bone mass, leveling off at two-thirds of original bone mass at 16 months after injury,4 chronic immobilization is known to cause a slowly progressing bone loss years after injury.2, 3, 16 Stratification of our participants by duration of injury or completeness of injury (Frankel grade) was performed to determine if either of these variables had an effect upon bone loss after the menopause, such association was not found. Thus, the fairly large magnitude in bone loss of 7.6% in the femoral neck and 5.7% in the trochanter appears primarily to be the result of menopause. The reason for the less apparent decline in BMD of the proximal tibia could be the already low values here compared to the other bone sites.1, 2, 4, 17 Again, the results were not statistically significant, and as such the above-mentioned thoughts must be considered hypothetical.
The bone mineral content of the spine was unchanged during early menopause in the SCL females of this study. Preservation of normal spine BMD in persons with SCL has been seen in many studies,1, 2, 3, 16, 17, 18, 19 as opposed to findings in the general older female population and in patients with endocrine disorders, who do lose bone in the spine.20 In individuals with SCL, however, secondary progressive skeletal abnormalities can lead to significantly increased vertebral bone densitometry results and thereby obscure an underlying osteoporosis.21
In normal early postmenopausal females, the change in biochemical markers of bone turnover correlates well with the changes in BMD in spine and forearm, and increases 50–100% from baseline values.22 Also in the present study, bone turnover increased in the years after the menopause. The increase reached significance for variables of bone formation, but not for variables of bone resorption. Confounders are the small number of participants and range between 0 and 7 years after the menopause. Still we see a clear tendency of loss in BMD, suggesting that bone resorption must increase more than bone formation as is the case in postmenopausal able-bodied women.
In conclusion, SCL subjects seem to lose bone in the femoral neck, but not in the spine and tibia, during and after menopause to the same extent as normal postmenopausal individuals. Biochemical markers of bone turnover increase after the menopause of SCL women as is also seen in postmenopausal able-bodied women. Our findings may encourage physicians to use prophylactic antiosteoporotic treatment at the time of menopause in SCL females, as a part of the life-long treatment strategy, which takes into consideration the whole situation of SCL females. Further studies with participation of more women are needed to confirm the findings and hypothesis of the present work.
References
Biering-Sørensen F, Bohr H, Schaadt O . Bone mineral content of the lumbar spine and the lower extremities years after spinal cord lesion. Paraplegia 1988; 26: 298–301.
Biering-Sørensen F, Bohr H, Schaadt O . Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest 1990; 20: 330–335.
Baumann WA, Spungen AM, Wang J, Pierson RN, Schwarz E . Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporosis Int 1999; 10: 123–127.
Garland DE, Stewart CA, Adkins RH . Osteoporosis after spinal cord injury. J Orthop Res 1992; 10: 371–378.
Roberts D et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 1998; 83: 415–422.
Smith DM, Khairi MRA, Johnston Jr CC . The loss of bone mineral with aging and its relationship to risk of fracture. J Clin Invest 1975; 56: 311–318.
Wasnich RD, Ross PD, Heilbrun LK, Vogel JM . Prediction of postmenopausal fracture risk with use of bone mineral measurements. Am J Obstet Gynecol 1985; 153: 745–751.
Vestergaard P, Krogh K, Rejnmark L, Mosekilde L . Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord 1998; 36: 790–796.
Gotfredsen A, Nilas L, Riis BJ, Thomsen K, Christiansen C . Bone changes occurring spontaneously and caused by oestrogen in early postmenopausal women: a local or generalised phenomenon? BMJ 1986; 292: 1098–1100.
Hansen MA, Overgaard K, Christiansen C . Spontaneous postmenopausal bone loss in different skeletal areas-followed up for 15 years. J Bone Miner Res 1995; 10: 205–210.
Minaire P, Berard E, Meunier PJ, Edouard C, Goedert G, Pilonchery G . Effects of disodium dichloromethylene diphosphonate on bone loss in paraplegic patients. J Clin Invest 1981; 68: 1086–1092.
Chappard D et al. Effects of tiludronate on bone loss in paraplegic patients. J Bone Miner Res 1995; 10: 112–118.
Zehnder Y et al. Prevention of bone loss in paraplegics over 2 years with alendronate. J Bone Miner Res 2004; 19: 1067–1076.
Sniger W . Alendronate increases bone density in chronic spinal cord injury: a case report. Arch Phys Med Rehab 2002; 83: 130–140.
Frankel HL et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia 1969; 7: 179–192.
Garland DE, Adkins RH, Rah A, Stewart CA . Bone loss with ageing and the impact of SCI. Top Spinal Cord Injury Rehab 2001; 6: 47–60.
Mohr T, Pødenphant J, Biering-Sørensen F, Galbo H, Thamsborg G, Kjær M . Increased bone mineral density after prolonged electrically induced cycle training of paralysed limbs in spinal cord injured man. Calcif Tissue Int 1997; 61: 22–25.
Wood DE, Dunkerley AL, Tromans AM . Results from bone mineral density scans in twenty-two complete lesion paraplegics. Spinal Cord 2001; 39: 145–148.
Szollar SM, Martin EM, Parthemore JG, Sartoris DJ, Sartoris DJ . Densitometric patterns of spinal cord injury associated bone loss. Spinal Cord 1997; 35: 374–382.
William DL, Nance PW . Dissociated hip and spine demineralisation: a specific finding in spinal cord injury. Arch Phys Med Rehab Sept 1993; 74: 960–964.
Jaovisidha S, Sartoris DJ, Martin EME, De Maeseneer M, Szollar SM, Deftos LJ . Influence of spondylopathy on bone densitometry using Dual energy X-ray absorptiometry. Calcif Tissue Int 1997; 60: 424–429.
Riis BJ, Overgaard K, Christiansen C . Biochemical markers of bone turnover to monitor the response to postmenopausal hormone replacement therapy. Osteoporosis Int 1995; 5: 276–280.
Acknowledgements
The study was financially supported by Det Lægevidenskabelige Forskningsfond for Grønland, Færøerne og Storkøbenhavn, Grosserer HC Andersen and Hustru Fond and Gigtforeningen. The statistical analysis on repeated measures analysis of variance was performed by Peter Dalgaard, Department of Biostatistics, University of Copenhagen. Secretary Lisbeth Nielsen and bioanalytical assistants Lone Bredahl and Marianna Thomson are thanked for practical assistance all through the study. Sally Kendall is thanked for improving the manuscript linguistically.
Author information
Authors and Affiliations
Additional information
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research
Rights and permissions
About this article
Cite this article
Broholm, B., Pødenphant, J. & Biering-Sørensen, F. The course of bone mineral density and biochemical markers of bone turnover in early postmenopausal spinal cord-lesioned females. Spinal Cord 43, 674–677 (2005). https://doi.org/10.1038/sj.sc.3101788
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101788