Abstract
Objective:
Hemodynamic infarction of the spinal cord that affected an 81-year-old female having a dissecting aortic aneurysm is presented. During the graft replacement operation, systemic hypotension occurred and the patient was subsequently complicated with paraplegia of the lower limbs. The patient died 2 weeks after the surgery due to gastrointestinal bleeding. An autopsy, which did not include the brain, was performed and the spinal cord was sampled. The aim of this report is to describe the pathologic profile of the spinal cord of the patient and to gain insight into the pathogenesis of the lesion.
Methods:
Histochemical and immunohistochemical methods were employed to study the spinal cord ranging from the lower thoracic to sacral segments.
Results:
The whole central areas of the spinal cord showed coagulation and/or liquefaction necroses, while the white matter on the circumference of the cord remained unaffected, thus exhibiting a ‘ring-like’ appearance.
Conclusion:
This case is an example of hemodynamic infarction of the spinal cord involving the gray matter that is supplied by the central artery, plus the border-zone that is supplied by both the central and peripheral arteries. The former is probably associated with selective vulnerability of the gray matter to ischemia, while the latter is probably associated with intrinsic vulnerability of the border-zone to systemic hypotension or low blood-flow states.
Similar content being viewed by others
Introduction
Hemodynamic infarction of the spinal cord has been a sporadic issue in the medical literature;1, 2, 3, 4, 5, 6 however, its pathologic profiles have not been sufficiently compared to the normal spinal arterial vasculature, due partly to the limited number of cases and/or its topographic diversity. Recently, in addition to the well-known ‘classic’ scheme that subdivides spinal arteries into the anterior spinal artery and posterior spinal arteries,5, 7 a ‘second’ scheme that subdivides spinal arteries into the following two arteries has been advocated: the central artery and the peripheral arteries.8, 9, 10 In this report, we describe the pathologic profiles of a case of hemodynamic infarction of the spinal cord and attempt to explain the pathogenesis of the lesion based on the above-mentioned ‘second’ scheme for subdivision of the spinal arteries.
Case report
An 81-year-old female complained of sudden back pain. Her medical history was unremarkable except that she had suffered from angina for the past few years. She was taken to a hospital by ambulance and was diagnosed as having acute dissecting aortic aneurysm (Stanford A or DeBakey I). An emergency graft replacement operation was undertaken, during which a drop in the blood pressure (the lowest of which was approximately 40/30 mmHg), the cause of which remained undetermined, occurred and resulted in a drop in the blood flow to the lower limbs. The left subclavian artery was bypassed to the bilateral femoral arteries to manage this problem. Right after the operation, the patient showed signs of paraplegia. Her neurological status was examined by a neurologist a few days post operation. She was drowsy and while her upper limbs showed some movements, her lower limbs were totally paraplegic. Although the deep tendon reflexes were present in the upper limbs, no deep tendon reflexes could be observed in the lower limbs. No response to plantar stimulation could be elicited. The sensory abnormalities seemed to be limited to the lower limbs, since she responded, with a grimace or some movements of the upper limbs, to pinprick to the trunk, but not to that to the lower limbs.
The subsequent clinical course was complicated with anuria and bacterial infection of the mediastinum. The patient died of gastrointestinal bleeding about 2 weeks after the surgery. An autopsy, which did not include the brain, was performed 2 h postmortem. The spinal cord was sampled from the lower thoracic to sacral segments.
Materials and methods
The spinal cord was fixed in 10% buffered formalin. The cord was serially dissected and the full length of the cord was sacrificed to prepare paraffin-embedded blocks. Sections were sliced and processed for hematoxylin and eosin (HE) and luxol fast blue/periodic acid Schiff (LFB/PAS) stains for a routine histological examination. An immunohistochemical stain was applied using antibodies to CD68 (PG-M1; 1:100; Dako, Carpinteria, CA, USA), glial fibrillary acidic protein (GFAP) (polyclonal; 1:1600; Dako) and neurofilament (anti-pan neurofilament; 1:50; Zymed, San Francisco, CA, USA). A Ventana autostainer (Ventana HX system; Benchmark; Tucson, AZ, USA) was employed for immunostaining using diaminobenzidine as the chromogen.
Results
Macroscopic results
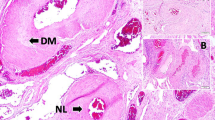
The dissection of the aorta ranged from the ascending aorta to the bifurcation of the descending aorta. The aorta showed severe atherosclerosis. The spinal cord was softened in its central portion from the lumbar through sacral segments (Figure 1). The artery of Adamkiewicz was not identified.
Microscopic results
Coagulation and/or liquefaction necroses were evident from approximately the first lumbar segment (L1) downward, suggestive of infarction of the spinal cord (Figure 2a). Many microglia and/or macrophages infiltrated the infarcted areas (Figure 2b). The intersegmental and intrasegmental distribution of the infarction is shown in Figure 3. The lower thoracic segments were unremarkable. In the uppermost segments involved (L1), the gray matter was selectively infarcted in such a manner that, while its approximately ventral two-thirds was infarcted, the posterior horn was well preserved. In the other segments downward, the infarction occupied the whole central areas of the cord. In contrast, the white matter on the circumference of the cord remained unaffected, thus exhibiting a ‘ring-like’ appearance. Many microglia and/or macrophages rimmed the infarcted areas. In the lowermost segments involved (lower sacral segments), the infarction showed a tendency to be confined to the gray matter again. In all the segments explored, atherosclerotic changes, thrombi, or emboli were absent in both the anterior and posterior spinal arteries.
There was no or mild astrocytic gliosis in the unaffected, ‘ring-like’ white matter, from which some GFAP-positive processes were stretched inwardly into the infarcted areas. There was mild reduction of neurofilament-positive axons in the ‘ring-like’ white matter. Axonal swelling was also a finding especially in the ventral part of the cord. Anterior horn cells that were positive for neurofilament and yet necrotic on HE-stained sections were present as well.
Discussion
The blood supply of the spinal cord comes from two intrinsic spinal arteries: the anterior spinal artery and the posterior spinal arteries.5, 7 The ventral two-thirds of the cord is supplied by the anterior spinal artery and the dorsal one-third of the cord is supplied by the posterior spinal arteries.7 The occlusion of these arteries leads to two well-known ischemic injuries, the syndrome of the anterior spinal artery and the syndrome of the posterior spinal arteries.11, 12, 13 Generally speaking, the topographic distribution of infarcts in these syndromes corresponds well to that of the two arteries.12, 13, 14 In the present patient, the infarction occupied the whole central areas of the cord with a rim of white matter unaffected on the circumference of the cord. This pattern is unexplainable by the occlusion of the two arteries.
A ‘second’ scheme for subdivision of spinal arteries has been advocated recently, in which the spinal arteries are subdivided into the following two arteries: the central artery and the peripheral arteries.8, 9, 10 The central artery is a branch of the anterior spinal artery, which, centrally oriented, runs through the anterior median sulcus, enters the cord and supplies most of the gray matter and the adjacent white matter. The peripheral arteries are anastomosing pial arterial branches irrigated by the anterior and posterior spinal arteries. They run around the cord and supply the posterior horn and the white matter on the circumference of the cord.
Located in between, there exists a border-zone that is supplied by both the central and peripheral arteries. Based on the topography of the spinal infarction of the present patient in conjunction with this ‘second’ scheme, the lesion could be interpreted as occupying the gray matter that is supplied by the central artery as well as the border-zone that is supplied by both the central and peripheral arteries.
The gray matter of the central nervous system is known to be susceptible to ischemia, while the white matter is known to be resistant.15 The border-zone of the spinal cord is probably as much a target for ischemic damage in systemic hypotension or low blood-flow states as is the supratentorial counterpart.16 Clinically, our patient had an episode of systemic hypotension during the course of operation and was thereafter complicated with paraplegia. Taken together, the pathogenesis of the spinal infarction of the patient is hypothesized as follows: the patient suffered from hemodynamic infarction of the spinal cord involving the gray matter as well as the border-zone between the central and peripheral arteries, the former probably being associated with selective vulnerability of the gray matter to ischemia and the latter probably being associated with intrinsic vulnerability of the border-zone to systemic hypotension or low blood-flow states. The white matter on the circumference of the cord, which topographically corresponds to areas supplied by the peripheral arteries alone, remained unaffected because of the resistance of white matter to ischemia. The scheme of this hypothesis is illustrated in Figure 4.
In reports dealing with experimental animal models of hypotensive ischemia or autopsied cases with or without dissecting aortic aneurysm, selective necrosis of the gray matter of the spinal cord has been described.1, 2, 15 This ‘selective necrosis’ of the gray matter is reputedly the most representative pathology of hypotensive ischemic injuries of the spinal cord.
In the present case, in the uppermost and lowermost spinal segments involved, the infarction was preferentially confined to the gray matter, without involving the white matter. In contrast, in the other segments involved that showed maximal injuries, the infarction involved not only the gray matter but also the white matter, resulting in the involvement of the border-zone as well. Given this observation as well as reviews of previous reports, it could be hypothesized as follows: the gray matter involvement is more than likely an essential pathologic substrate in hemodynamic infarction of the spinal cord, while the border-zone involvement is a chance accompaniment that is determined by factors such as the duration and severity of ischemia.15
The present case is not the first one of its kind. In a report dealing with a case of spinal infarction having a similar pathology to the present one,6 a hypothesis was suggested stating that the order of vulnerability of the spinal cord is: 1 gray matter; 2 white matter adjacent to gray matter; and 3 peripheral zone of white matter. This hypothesis is, albeit coincidentally, fairly consistent with ours. In another case by Hogan et al,3 a spinal infarction that was very similar to our present case was documented. The authors suggested that it was a case of hemodynamic infarction of the spinal cord involving primarily the border-zone, situated not between the central and peripheral arteries, but rather between the anterior and posterior spinal arteries. This hypothesis needs reconsideration, since it cannot account for either the involvement of the whole central areas of the cord or the ‘ring-like’ preservation of the white matter on the circumference of the cord. As was suggested by this report and elsewhere,17 however, the presence of another border-zone, that is, one between the anterior and posterior spinal arteries, may be true as well. Probably, there are two border-zones in the spinal cord: one between the central and peripheral arteries and the other between the anterior and posterior spinal arteries. This hypothesis may be important when interpreting the multifarious pathologic profiles of infarction of the spinal cord.
References
Kepes JJ . Selective necrosis of the spinal cord gray matter. A complication of dissecting aneurysm of the aorta. Acta Neuropathol 1965; 4: 293–298.
Herrick MK, Mills Jr PE . Infarction of spinal cord. Two cases of selective gray matter involvement secondary to asymptomatic aortic disease. Arch Neurol 1971; 24: 228–241.
Hogan EL, Romanul FCA . Spinal cord infarction occurring during insertion of aortic graft. Neurology 1966; 16: 67–74.
Blumbergs PC, Byrne E . Hypotensive central infarction of the spinal cord. J Neurol Neurosurg Psychiatry 1980; 43: 751–753.
Girolami UD, Frosch MP, Tator CH . Regional neuropathology: diseases of the spinal cord and vertebral column. In: Graham DI, Lantos PL (eds). Greenfield's Neuropathology 7th edn. Arnold: London 2002 Chapter 18, pp 1063–1101.
Thompson GB . Dissecting aortic aneurysm with infarction of the spinal cord. Brain 1956; 79: 111–117.
Gillilan LA . The arterial blood supply of the human spinal cord. J Comp Neurol 1958; 110: 75–103.
Hassler O . Blood supply to human spinal cord. Arch Neurol 1966; 15: 302–307.
Turnbull IM, Breig A, Hassler O . Blood supply of cervical spinal cord in man: a microangiographic cadaver study. J Neurosurg 1966; 24: 951–966.
Turnbull IM . Microvasculature of the human spinal cord. J Neurosurg 1971; 35: 141–147.
Steegmann AT . Syndrome of the anterior spinal artery. Neurology 1952; 2: 15–35.
Hughes JT . Thrombosis of the posterior spinal arteries. A complication of an intrathecal injection of phenol. Neurology 1970; 20: 659–664.
Hughes JT, Brownell B . Cervical spondylosis complicated by anterior spinal artery thrombosis. Neurology 1964; 14: 1073–1077.
Laguna J, Cravioto H . Spinal cord infarction secondary to occlusion of the anterior spinal artery. Arch Neurol 1973; 28: 134–136.
DeGirolami U, Zivin JA . Neuropathology of experimental spinal cord ischemia in the rabbit. J Neuropathol Exp Neurol 1982; 41: 129–149.
Auer RN, Sutherland GR . Hypoxia and related conditions. In: Graham DI, Lantos PL (eds). Greenfield's Neuropathology 7th edn. Arnold: London 2002 Chapter 5, pp 233–280.
Sandson TA, Friedman JH . Spinal cord infarction. Report of 8 cases and review of literature. Medicine 1989; 68: 282–292.
Acknowledgements
We thank Mr T Mochino and Mr T Honma for their excellent technical assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ishizawa, K., Komori, T., Shimada, T. et al. Hemodynamic infarction of the spinal cord: involvement of the gray matter plus the border-zone between the central and peripheral arteries. Spinal Cord 43, 306–310 (2005). https://doi.org/10.1038/sj.sc.3101671
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101671
Keywords
This article is cited by
-
Ischemic damage may play an important role in spinal cord injury during dancing
Spinal Cord (2020)
-
Systemic Complications of Spinal Cord Injury
Current Neurology and Neuroscience Reports (2017)
-
Anterior disco-osteo-arterial conflict as a cause of intersegmental arterial flow impairment and spinal cord ischemia
Neuroradiology (2016)
-
Delayed post-traumatic spinal cord infarction in an adult after minor head and neck trauma: a case report
Journal of Medical Case Reports (2012)
-
Spinal Cord Infarction with Multiple Etiologic Factors
Journal of General Internal Medicine (2007)