Abstract
Study design: Survey.
Objective: Determine intramuscular fat (IMF) in affected skeletal muscle after complete spinal cord injury using a novel analysis method and determine the correlation of IMF to plasma glucose or plasma insulin during an oral glucose tolerance test.
Setting: General community of Athens, GA, USA.
Methods: A total of 12 nonexercise-trained complete spinal cord injured (SCI) persons (10 males and two females 40±12 years old (mean±SD), range 26–71 years, and 8±5 years post SCI) and nine nonexercise-trained nondisabled (ND) controls 29±9 years old, range 23–51 years, matched for height, weight, and BMI, had T1 magnetic resonance images of their thighs taken and underwent an oral glucose tolerance test (OGTT) after giving consent.
Results: Average skeletal muscle cross-sectional area (CSA) (mean±SD) was 58.6±21.6 cm2 in spinal cord subjects and 94.1±32.5 cm2 in ND subjects. Average IMF CSA was 14.5±6.0 cm2 in spinal cord subjects and 4.7±2.5 cm2 in nondisabled subjects, resulting in an almost four-fold difference in IMF percentage of 17.3±4.4% in spinal cord subjects and 4.6±2.6% in nondisabled subjects. The 60, 90 and 120 min plasma glucose or plasma insulin were higher in the SCI group. IMF (absolute and %) was related to the 90 or 120 min plasma glucose or plasma insulin (r2=0.71–0.40).
Conclusions: IMF is a good predictor of plasma glucose during an OGTT and may be a contributing factor to the onset of impaired glucose tolerance and type II diabetes, especially in SCI. In addition, reports of skeletal muscle CSA should be corrected for IMF.
Similar content being viewed by others
Introduction
Observations of magnetic resonance images (MRI) revealed that intramuscular fat (IMF) and subfascial fat appeared to be increased in chronic spinal cord injured (SCI) patients when compared with nondisabled (ND) controls. Goodpaster et al1, 2, 3 demonstrated that subcutaneous, subfascial and intramuscular fat (IMF) of lean or obese subjects could relate to euglycemic clamp insulin sensitivity. IMF was correlated with insulin sensitivity (r=−0.45), while subfascial and subcutaneous fat had little relation, r=−0.36 and −0.12, respectively. The concept that fat could impede sugar metabolism in skeletal muscle could be a mechanism for the 1 million new diabetes cases each year in those 20 years of age or older4 in the United States. In fact, diabetes is the fifth leading cause of death in the United States, responsible for 212,000 deaths annually (US government statistics).
Inactivity probably plays a role in insulin resistance. Individuals with SCI occupy the lowest end of the physically activity spectrum, and there is an increased prevalence of insulin resistance and type-II diabetes in this population. Bauman and Spungen5 demonstrated that individuals with SCI had elevated plasma glucose 90 or 120 min after and elevated plasma insulin 120 min after a 75 g oral glucose load. Elevations in glucose and insulin were less in ND controls. From this cross-sectional design, impaired glucose tolerance and type II diabetes appeared to occur more often after SCI.
An additional issue concerns increases in fat accumulation in the thighs and the reliability and validity of cross-sectional area (CSA) measurements. If individuals with SCI have increased fat accumulation in the thighs after injury that is not quantified and corrected, the result is a potentially large overestimation of CSA. Responses to training or other interventions could also be unreliable if changes occur in fat CSA.
The relation between regional fat distribution in the thighs and oral glucose tolerance has never been investigated in individuals with SCI. This study was conducted to examine the distribution of fat in the thighs of individuals with complete SCI and ND individuals and investigate the relation of these fat stores to oral glucose tolerance. The aim was also to provide evidence that inactivity and the resulting IMF accumulation are related to the development of impaired glucose tolerance and type II diabetes. A secondary purpose was to support the concept that IMF accumulation needs to be quantified and corrected for when measuring skeletal muscle CSA.
Methods
Subjects
In all, 10 male and two female subjects, nonexercised trained, complete SCI individuals were recruited from Shepherd Center (Atlanta, GA, USA). Three male and six female subjects, also nonexercised trained, were recruited as ND controls from the student population of The University of Georgia and the staff of Shepherd Center. All controls had voluntary motor control and somatic sensation in both lower and upper limbs. Each provided written informed consent after the study was explained. The Human Research Review Boards of the Shepherd Center and The University of Georgia approved the study. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Magnetic resonance imaging (MRI) and analysis of thigh composition
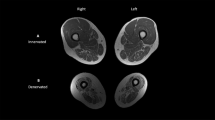
T1-weighted transaxial images of both thighs were collected at a field strength of 1.5 T with a Sigma imaging system (General Electric, Milwaukee, WI, USA), utilizing a 40 cm field of view and a 256 × 256 matrix. Individual cross sections were 1 cm thick and 1 cm apart. Analysis of the thigh began with the first image distal to the gluteal muscle and proceeded distally to the image just proximal to the appearance of the patella. Analysis of the images were performed using the Xvessel software (Ron Meyer, PhD at Michigan State University).
Sample MR images showing partial analysis procedures are presented in Figures 3 and 4. Images were automatically segmented into fat, skeletal muscle and background/bone regions. A preliminary segmentation of each original 2D slice into fat versus nonfat was obtained by simplex optimization of the correlation between a Sobel-gradient image computed from the original image6 versus the corresponding gradient image computed from single-threshold fat-segmented image. This first-pass segmentation was used to correct for intensity variations across the original image caused by RF heterogeneity, as suggested by Cohen et al.7 The corrected original image was then resegmented into the three aforementioned components using a fuzzy c-mean clustering algorithm.8 Manual selection of any pixel of skeletal muscle subsequently highlighted all skeletal muscle pixels in the region and provided a total number of skeletal muscle pixels exclusive of any fat or low pixels. A manual tracing was drawn around the outside edge of the highlighted skeletal muscle region (including the fascial border) to create a new region of interest (ROI). The ROI contained skeletal muscle, subfascial fat, intramuscular fat and low-intensity pixels. A second manual tracing was drawn around the skeletal muscle under the fascia to differentiate between subfascial and IMF. A manual tracing was also drawn around the femur in order to exclude it from the ROI. The total number of pixels within the ROI and a frequency distribution and histogram of all pixels and signal intensities were produced. A representative histogram is presented in Figure 2. To differentiate between skeletal muscle and fat in the histogram, a midpoint between skeletal muscle and fat peaks was chosen. Midpoint values corresponded to an average signal intensity of 378 (arbitrary units). Skeletal muscle pixels were ⩽378 and fat pixels were >378. The value calculated as skeletal muscle pixels from the histogram was the sum of the skeletal muscle and low-intensity pixels. The values used in the final calculation of percent fat were (1) skeletal muscle pixels from segmentation, (2) fat pixels from the histogram and (3) a total region that represented the sum of (1) and (2) and low pixels. The individual collecting the data performed two trials on 20 sample images (half from ND and half from SCI) to determine test–retest reliability. Scores were r=0.99 for skeletal muscle pixels from segmentation, r=0.98 for fat pixels from the histogram and r=0.99 for the whole region.
Glucose tolerance test and insulin assay
Following an overnight fast, subjects reported to the laboratory at 0900 h and each was administered a 75 g oral glucose load within 5 min. Blood samples were collected immediately before, and 60, 90 and 120 min after glucose ingestion for measurement of plasma glucose and plasma insulin. Blood for plasma glucose determination was collected with sodium fluoride and blood for plasma insulin with heparin. Blood was either immediately centrifuged and separated for analysis or frozen at −20°C until it could be analyzed. Plasma glucose assays were performed on an automated glucose analyzer and plasma insulin by radioimmunoassay.
Statistical analysis
SCI and ND subjects were examined as independent groups or pooled into one group for regression analyses as needed. Independent t-tests were conducted to detect between group differences. Nonparametric tests for two independent groups were also performed to account for the possibility that SCI individuals represent a non-Gaussian population. Simple regression analyses were performed with plasma glucose and plasma insulin during the glucose tolerance test as the dependent variables and measures of thigh composition as the predictor variables. All analyses were conducted in the SPSS version 10.0.
Results
Characteristics
Subject characteristics are presented in Table 1. SCI subjects were significantly older than ND subjects (42±12, range 26–71 versus 29±9, range 20–54, respectively), but they were similar in height and weight. SCI subjects' level of injury ranged from C-3 to C-7 and duration of injury was 8±5 years (range 1–16 years). All SCI subjects were classified as ASIA A complete.
Thigh skeletal muscle and fat
Muscle and fat CSA and percent composition are presented in Table 2. ND subjects had more skeletal muscle CSA than the SCI subjects and those with SCI had over three times more IMF and almost four times more subfascial fat. Consequently, SCI subjects also had almost a four-fold greater percentage of intramuscular and subfascial fat in the thigh.
Glucose tolerance tests
Glucose and insulin responses to the oral glucose tolerance test (OGTT) are presented in Table 3. Fasting plasma glucose and plasma insulin were not different between SCI and ND groups. SCI subjects had greater 60, 90 and 120 min plasma glucose and plasma insulin during the OGTT. Taking the difference between fasting plasma glucose and levels at the other time points, SCI subjects had greater increases in plasma glucose at 60, 90 and 120 min during the glucose tolerance test than ND subjects. Plasma insulin levels showed the same elevations in SCI subjects.
Correlation of glucose and insulin to fat
Significant r2 values were found between regional fat content or percentage fat in the thigh and plasma glucose or plasma insulin during the OGTT (Figure 1). These r2 values were identical in both independent groups and when the data were pooled. The IMF % predicted the 90 and 120 min plasma glucose (r2=0.52 and 0.71, respectively, P<0.001). The plasma insulin at 120 min was also estimated (r2=0.41). IMF predicted both the 120 min plasma glucose and plasma insulin (r2=0.44 and 0.59, respectively, P<0.001).
Discussion
This study used MRI and a novel analysis method to noninvasively quantify regional fat distribution and its correlation to an OGTT in SCI and ND controls. The data show the disparity in skeletal muscle CSA between ND and SCI very clearly. More importantly, the data show the large difference between groups in fat accumulation and distribution, especially IMF. In addition to high IMF, SCI subjects also had higher glucose and insulin values than controls at all points after glucose ingestion during the OGTT. Simple regression analyses show the strongest relationships between IMF percentage and the 90 and 120 min plasma glucose levels.
SCI and ND groups were not different in height, mass, and BMI. They were different in age and gender, the SCI group was older and had more males. Age and gender have been shown to have an effect on total body composition. Older or female populations tend to have more total fat than those who are younger or male.9, 10, 11 This is a limitation of this investigation. Nonparametric statistics conducted to test between group differences provide the same results as the parametric methods. With nonparametric methods, the SCI and ND samples are not different in height, mass, or BMI, but they are different in age. However, the distribution of IMF and the effect of gender and age have not been established in the populations examined in this study using the current novel analysis method. This is certainly an area for future research using this method.
The histogram (pixel number versus signal intensity) produced from analysis of the quadriceps femoris muscle group within a thigh MRI showed two distinct regions (Figure 2). The peak on the left was distinct, thin, and represented skeletal muscle. The peak on the right was the peak representing fat. It was also distinct and thin. Most of the pixels were in one or the other of these two distinct peaks that were signal intensity units apart, making it simple (pixel CSA times number of pixels) to determine the skeletal muscle and fat CSA. Test–retest reliability was r=0.99 for muscle and fat areas examined in both ND and SCI groups.
Several investigators have used magnetic resonance spectroscopy (MRS) to quantify intramyocellular and extramyocellular lipid.12, 13, 14 Jacob et al,13 compared intra- and extramyocellular lipid in the tibialis anterior and soleus skeletal muscles between ND insulin-sensitive and insulin-resistant groups matched for age, sex, BMI, percent body fat, VO2max, and waist-to-hip ratio. They found intramyocellular lipid to be increased in the insulin-resistant group. Contrary to the present study, extramyocellular lipid, which is the closest comparable fat measurement to those presented here, was not different between groups. MRS methodology12, 13, 14 is built upon measurements that come from only one or two representative regions rather than the whole body segment, possibly disguising or creating differences between groups. It is also possible that the absolute amount and distribution of fat (extramyocellular) in the smaller tibialis anterior and soleus could have less of an impact on insulin resistance than the larger thigh muscles.
Our measurements of IMF with MRI are consistent with the computed tomography measurements of the thigh by Goodpaster et al,1, 2, 3 although our correlations are much stronger. Computed tomography is subject to some of the same limitations as MRS in that data is only collected from a very limited number of slices of the body segment (Figures 3 and 4). Goodpaster examined ND lean and obese subjects, possibly limiting the strength of the correlations by only showing the contribution of fat accumulation and not also including muscle atrophy. SCI individuals also have an extreme degree of atrophy. There is an equivalent inverse correlation between percent skeletal muscle and 2 h plasma glucose of r2=−0.67, suggesting that loss of skeletal muscle mass and the accumulation of IMF have almost equal parts in abnormal disposal of glucose. SCI individuals appear to experience both factors simultaneously, whereas ND individuals who are simply obese may not experience a loss of skeletal muscle mass as their weight increases. We have reported 50% atrophy of the quadriceps femoris muscle group in SCI without correcting for IMF.15, 16
Our data support the idea that lipid could interfere with sugar metabolism in skeletal muscle, although a clear mechanism has not been established. Palmitoyl CoA arising from lipid metabolism could inhibit PFK and disturb the gradients for sugar entry in skeletal muscle.17, 18 The data also support the notion that inactivity plays a role in the etiology of impaired glucose tolerance and type II diabetes. The strong relations between plasma glucose and IMF exist within the muscles directly affected by inactivity as a result of SCI.
The data presented here suggest that IMF increases dramatically after SCI. Current methods estimate that skeletal muscle CSA of the quadriceps decreases from 103 to 49 cm2 6 months after SCI.15 By accounting for increases in IMF using the results of this study, the actual atrophy of skeletal muscle would be closer to 65% than 50%. Similar errors may occur with measurements of changes in muscle CSA in response to resistance training. Actual muscle CSA may be over- or underestimated depending on the state of IMF before and after training. Training studies using MRI should be evaluated in this regard.
In summary, this study showed with MRI that IMF is a good predictor of plasma glucose during an OGTT. Lipid around skeletal muscle accounts for about 70% of the variance in plasma glucose 120 min after ingesting a 75 g glucose in individuals with SCI and those who are ND. The strong prediction of 2-h plasma glucose appears to be due to both IMF accumulation and loss of skeletal muscle mass caused by SCI. This is a major health concern for those who have SCI and other diseases that cause atrophy and increased inactivity, including the sedentary lifestyle. Measurement of IMF is essential for accurate representation of CSA. Failure to account for IMF can cause an underestimation of skeletal muscle size. MRI analysis of IMF could be a tool to noninvasively assess the risk of impaired glucose tolerance and type II diabetes. Future studies should be conducted to further refine this technique and to investigate the mechanistic relationship between IMF and blood glucose.
References
Goodpaster BH, Theriault R, Watkins SC, Kelley DE . Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 2000; 49: 467–472.
Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE . Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997; 46: 1579–1585.
Goodpaster BH, Thaete FL, Kelley DE . Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000; 71: 885–892.
Spungen AM, Wang J, Pierson Jr RN, Bauman WA . Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol 2000; 88: 1310–1315.
Bauman WA, Spungen AM . Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994; 43: 749–756.
Gonzalez RC, Wintz P . Digital Image Processing, 2nd edn. Addison-Wesley: Reading, MA, 1987.
Cohen MS, DuBois RM, Zeineh MM . Rapid and effective correction of RF inhomogeneity for high field magnetic resonance imaging. Hum Brain Mapp 2000; 10: 204–211.
Suckling J, Sigmundsson T, Greenwood K, Bullmore ET . A modified fuzzy clustering algorithm for operator independent brain tissue classification of dual echo MR images. Magn Reson Imaging 1999; 17: 1065–1076.
Von Dobeln W . Human standard and maximal metabolic rate in relation to fat-free body mass. Acta Physiol Scand 1956; 37(Suppl 126): 1–79.
Kohrt WM, Malley MT, Dalsky GP, Holloszy JO . Body composition of healthy sedentary and trained, young and older men and women. Med Sci Sports Exerc 1992; 24: 832–837.
Snead DB, Birge SJ, Kohrt WM . Age-related differences in body composition by hydrodensitometry and dual-energy X-ray absorptiometry. J Appl Physiol 1993; 74: 770–775.
Sinha R et al. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 2002; 51: 1022–1027.
Jacob S et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 1999; 48: 1113–1119.
Mayerson AB et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 2002; 51: 797–802.
Dudley GA, Castro MJ, Rogers S, Apple Jr DF . A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 1999; 80: 394–396.
Castro MJ, Apple Jr DF, Staron RS, Campos GE, Dudley GA . Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol 1999; 86: 350–358.
Boden G, Chen X, Ruiz J, White JV, Rossetti L . Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 1994; 93: 2438–2446.
Randle PJ . Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 1998; 14: 263–283.
Acknowledgements
We thank the subjects for volunteering for this study and NIH for supporting it (HD 39676 to GAD and AR 43903 to RAM).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elder, C., Apple, D., Bickel, C. et al. Intramuscular fat and glucose tolerance after spinal cord injury – a cross-sectional study. Spinal Cord 42, 711–716 (2004). https://doi.org/10.1038/sj.sc.3101652
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101652
Keywords
This article is cited by
-
Denervation impacts muscle quality and knee bone mineral density after spinal cord injury
Spinal Cord (2023)
-
Serum concentrations of ketones increase after hand-ergometer exercise in persons with cervical spinal cord injuries: a preliminary prospective study
Spinal Cord (2023)
-
Effects of functional electro-stimulation combined with blood flow restriction in affected muscles by spinal cord injury
Neurological Sciences (2022)
-
Co-contraction of ankle muscle activity during quiet standing in individuals with incomplete spinal cord injury is associated with postural instability
Scientific Reports (2021)
-
Bioinformatic analysis of the gene expression profile in muscle atrophy after spinal cord injury
Scientific Reports (2021)