Abstract
Study design: Preliminary longitudinal clinical trial.
Objectives: To test the efficacy of repetitive transcranial magnetic stimulation (rTMS) in modulating corticospinal inhibition and improving recovery in stable incomplete spinal cord injury (iSCI).
Setting: National Spinal Injuries Centre, Stoke Mandeville Hospital, Bucks, UK and Division of Neuroscience, Imperial College Faculty of Medicine, Charing Cross Hospital, London, UK.
Methods: Four stable iSCI patients were treated with rTMS over the occipital cortex (sham treatment) and then over the motor cortex (real treatment). Patients were assessed using electrophysiological, clinical and functional measures before treatment, during sham treatment, during the therapeutic treatment and during a 3-week follow-up period.
Results: Cortical inhibition was reduced during the treatment week. Perceptual threshold to electrical stimulation of the skin, ASIA clinical measures of motor and sensory function and time to complete a peg-board improved and remained improved into the follow-up period.
Conclusion: In this preliminary trial, rTMS has been shown to alter cortical inhibition in iSCI and improve the clinical and functional outcome.
Sponsorship: This work was supported by the International Spinal Research Trust.
Similar content being viewed by others
Introduction
Patients who recover motor function following incomplete spinal cord injury (iSCI) exhibit a natural downregulation of inhibition within the motor cortex that may encourage motor recovery by promoting increased cortical drive to surviving corticospinal neurones.1, 2 This change occurs within a month of injury3 and has been identified in patients with iSCI in the cervical spinal cord by recording electromyographic (EMG) responses in hand muscles to single-pulse transcranial magnetic stimulation (TMS) of the motor cortex. Such downregulation of cortical inhibition might not only be associated with natural recovery following iSCI; it may also be a useful mechanism to promote active recovery of motor function. Repetitive transcranial magnetic stimulation (rTMS) can modulate corticospinal inhibition within the motor cortex of healthy individuals.4 We have now used a novel rTMS protocol in iSCI patients in an attempt to reduce inhibition further and improve function.
Methods
With local ethical committee approval, four stable iSCI patients (three male (ages 41, 54 and 54 years; duration of injury 7–8 years); one female (age 26 years; duration of injury 15 months)) were recruited from the out-patients clinic. All patients had their clinical level of lesion diagnosed at C5 and all had D-gradings when assessed according to American Spinal Injury Association (ASIA) criteria.5 We used a MagStim 200 magnetic stimulator (The Magstim Company; UK) connected to a circular (9 cm average diameter) stimulating coil. The stimulus intensity was set to 90% threshold for evoking motor evoked potentials (MEPs) in hand muscles and was assessed with the coil centred over the vertex with a clockwise current-flow in the brain to activate the left motor cortex. Patients received 5 days of sham rTMS (360 doublet pulses each) delivered over the occipital cortex followed by 5 days of therapeutic stimulation delivered over the motor cortex. Double pulses of rTMS were applied separated by 100 ms (10 Hz) at a frequency of 0.1 Hz (10 s interval). Each rTMS treatment took place over five consecutive days with each session lasting for 1 h (a total of 360 doublet pulses). Patients were assessed using electrophysiological, clinical and functional measures before treatment, during sham treatment, during the therapeutic treatment and during a 3-week follow-up period. Electrophysiological assessments were conducted during weak voluntary contraction (5–10% maximum voluntary contraction) to allow investigation of corticospinal inhibition. TMS was applied to the left motor cortex to produce MEPs and periods of inhibition (silent periods) in the right (dominant hand) thenar muscles. In the first baseline testing session, the stimulus intensity for each patient was adjusted so that a cortical silent period could be identified in the on-going EMG in the absence of an MEP. This intensity was used for that patient in all subsequent testing sessions. Expressing the area of inhibition as a proportion of a fixed area (50 ms) of prestimulus, voluntary activity allowed us to derive an index of corticospinal inhibition. Perceptual threshold to electrical stimulation of the skin was assessed over the thenar muscles using the EMG recording electrodes as stimulating electrodes.6 Clinical assessments of sensory (pin-prick and light touch) and motor function were made according to ASIA criteria.5 Patients completed a timed nine-hole peg board with their dominant (all right) hand to assess overall function. Each patient underwent a total of 11 assessments as follows: baseline period two sessions; sham treatment week two sessions; therapeutic treatment week four sessions; follow-up period, weekly for 3 weeks.
Results
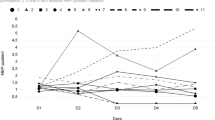
Patients showed no difference (unpaired ANOVA; P<0.05) between the baseline and sham week assessments, so these were combined into one data set before the week of therapeutic treatment. The mean (±SEM) level of intracortical inhibition was reduced to 37.5±8.0 % of pretreatment levels (baseline and sham combined) during the week of therapeutic treatment (P<0.05; ANOVA on ranks with Dunn's correction) and returned to 90.2±15% of pretreatment levels during the follow-up period (Figure 1a). This cortical change was associated with improvement in clinical measures of both motor (Figure 1b; P<0.05; ANOVA on ranks with Dunn's correction) and sensory function (light touch Figure 1c; not significant (P>0.05) and pin-prick Figure 1d; P<0.05; ANOVA with Tukey correction) of 4–10% during treatment week. Patients showed improved perceptual threshold6 to electrical stimulation of the skin (Figure 1e; P<0.05; ANOVA with Tukey correction; pretreatment 1.64±0.16 mA; treatment week 1.40±0.15 mA; follow-up 1.38±0.11 mA). Patients showed an improved ability to complete the peg-board test (Figure 1f; P>0.05 in treatment week and P<0.05 in follow-up week; ANOVA with Tukey correction; pretreatment 30.8±1.3 s; treatment week 27.0±1.3 s; follow-up week 25.4±1.3 s). These clinical and functional improvements persisted into the follow-up period.
Assessments of (a) cortical inhibition, (b) ASIA motor score, (c) ASIA light touch score, (d) ASIA pin-prick score, (e) perceptual threshold and (f) timed peg-board test; before treatment (baseline period and sham treatment combined), during therapeutic treatment and during the 3-week follow-up period. Asterisks indicate statistically significant difference (P<0.05)
Conclusions
The clinical changes observed are consistent with the idea that reduced corticospinal inhibition can facilitate functional recovery and this is reflected by the increase in ASIA sensory and motor scores, perceptual threshold and possibly the improved dexterity indicated by faster peg-board completion times. The changes we observed in sensory function are illustrated by reduced perceptual threshold to electrical stimulation and by increased ASIA sensory scores. There was no change in the clinical level and the increased ASIA scores reflected an improvement in sensation in several dermatomes below the lesion. These changes may have been driven by stimulation of sensory cortex since the rTMS coil is large enough to cover this as well as motor cortex. Previously, there has been little evidence that TMS can activate sensory cortex but there is one report of body sensations evoked by TMS in spinal cord injury patients;7 possibly sensory gating mechanisms are altered following the deafferentation that occurs as a result of spinal injury making the sensory cortex more amenable to stimulation by TMS. It is possible that rTMS can also affect plasticity within the sensory cortex and produce changed sensory thresholds in the patients in this study. The gamut of assessments was performed on 11 occasions over 7 weeks. None of the individual measurements showed any significant change over the four sessions (baseline period and sham treatment combined) before the week of therapeutic treatment which reduces the chance of the observed changes being related to practice. Although the timed peg-board test could have improved with practice, other studies have shown good test–retest reliability of pegboards as assessment tools for manual dexterity (eg Gallus and Mathiowetz8).
This preliminary study has, for the first time, shown that rTMS treatment in patients with chronic stable iSCI can produce reductions in corticospinal inhibition that can be detected using electrophysiological techniques. The positive clinical and functional changes associated with this treatment continue for at least several weeks after the electrophysiological change has reverted to pretreatment levels. This study makes rTMS a strong candidate for further development as a rehabilitation tool in spinal cord injury and further work on a larger population of patients with a double-blind crossover protocol will assert whether the beneficial changes were a direct result of the treatment procedure.
References
Davey NJ, Romaiguère P, Maskill DW, Ellaway PH . Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man. J Physiol 1994; 477: 223–235.
Davey NJ et al. Responses of thenar muscles to transcranial magnetic stimulation of the motor cortex in incomplete spinal cord injury patients. J Neurol Neurosurg Psychiatry 1998; 65: 80–87.
Smith HC et al. Neurological assessment and corticospinal function studied over time following incomplete spinal cord injury. Spinal Cord 2000; 38: 292–300.
Ziemann U, Corwell B, Cohen LG . Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci 1998; 18: 1115–1123.
Maynard Jr FM et al. International standards for neurological function classification of spinal cord injury. Spinal Cord 1997; 35: 266–274.
Davey NJ, Nowicky AV, Zaman R . Somatotopy of perceptual threshold to cutaneous electrical stimulation in man. Exp Physiol 2001; 186: 127–130.
Cohen LG, Topka H, Cole RA, Hallett M . Leg parathesias induced by magnetic brain stimulation in patients with thoracic spinal cord injury. Neurology 1991; 41: 1283–1288.
Gallus J, Mathiowetz V . Test–retest reliability of Purdue Pegboard for persons with multiple sclerosis. Am J Occup Ther 2003; 57: 108–111.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Belci, M., Catley, M., Husain, M. et al. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 42, 417–419 (2004). https://doi.org/10.1038/sj.sc.3101613
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101613
Keywords
This article is cited by
-
Intermittent theta burst stimulation modulates biceps brachii corticomotor excitability in individuals with tetraplegia
Journal of NeuroEngineering and Rehabilitation (2022)
-
Repetitive TMS in treatment of resistant diabetic neuropathic pain
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2019)
-
Effects of high-frequency transcranial magnetic stimulation on functional performance in individuals with incomplete spinal cord injury: study protocol for a randomized controlled trial
Trials (2017)
-
White matter changes in corticospinal tract associated with improvement in arm and hand functions in incomplete cervical spinal cord injury: pilot case series
Spinal Cord Series and Cases (2017)
-
Long-term paired associative stimulation can restore voluntary control over paralyzed muscles in incomplete chronic spinal cord injury patients
Spinal Cord Series and Cases (2016)