Abstract
Study design: Experimental laboratory investigation of the effect of anesthesia on evoked potentials in rats.
Objectives: To define the optimal ketamine/xylazine anesthesia levels for the recording of different evoked potentials.
Setting: BioSurgery Preclinical Department, Baxter BioScience, Vienna, Austria.
Methods: Rats were implanted with cranial screws that allow stimulation and recording of evoked potentials. Somatosensory evoked potentials (SEPs), brainstem-derived motor evoked potentials (BMEPs) and corticomotor evoked potential (CMEPs) were recorded under different levels of anesthesia. The recorded signals were evaluated by measuring their latencies and amplitudes. The level of anesthesia was assessed by scoring the hind limb withdrawal reflex.
Results: All three signals showed a strong dependency on the level of anesthesia. The observed effects, however, differed between the three signals. SEP amplitudes and latencies declined as animals slowly transgressed from deep to light anesthesia. In contrast, BMEP amplitudes were larger and latencies shorter in light anesthesia than in deep anesthesia. CMEPs finally were hard to record under deep anesthesia, but were easily recorded in light anesthesia. BMEPs that were recorded during light anesthesia also showed a significant change in configuration that was coupled with a notable increase in the variability of its amplitudes.
Conclusions: The level of ketamine/xylazine anesthesia affects evoked potentials and thus should be controlled during electrophysiological recording. Our results suggest that SEPs should be best recorded during deep anesthesia, while BMEPs and CMEPs are best recorded during intermediate and light anesthesia.
Similar content being viewed by others
Introduction
Motor evoked potentials (MEPs) and somatosensory evoked potentials (SEPs) reflect the functional integrity of the central nervous system (CNS) and therefore represent valuable diagnostic and prognostic tools in neurology, neurosurgery, and traumatology.1,2,3,4 Because of their clinical relevance, evoked potentials are also being used in experimental research. Various methods allow recording of brainstem-derived motor evoked potentials (BMEPs), corticomotor evoked potentials (CEMPs), and sensory evoked potentials (SEPs) in laboratory animals, in particular in the rat.5,6,7,8
While recording MEPs and/or SEPs has been a method of choice in many experimental studies of spinal cord injury, their diagnostic and/or prognostic value has not always been ascertained. In particular, several authors have found little correlation between residual MEP amplitude and functional recovery following graded SCI in the rat.7,9,10 One of the reasons for this lack in correlation may be the great variability in the shape and the size (amplitude) of the recorded signals. Indeed, different groups have reported electromyographic MEP amplitudes in healthy rats ranging from as low as 50 μV11 to more than 10 mV.7,12 Moreover, variability in MEP amplitudes has also been dramatic within studies.7,9,11,12
In a previous study we have found that controlling the depth of anesthesia will reduce the variability in SEP and BMEP amplitudes.8 This suggests that varying types and levels of anesthesia may underlie the variability in reported MEP and SEP amplitudes. This assumption is further supported by reports that show that anesthesia can have a marked effect on SEPs and MEPs in animals and humans.13,14,15,16,17,18 Specifically, volatile and barbiturate anesthetics have been shown to depress and/or abolish evoked potentials. Ketamine alone or in combination with xylazine, on the other hand, has little or no influence on evoked potentials.15,17 Moreover, ketamine in combination with xylazine has been shown in rats to result in sufficient anesthesia and analgesia without depressing vital functions.19,20,21
These results have led to the recommendation that ketamine be used in animal models of neurogenic disease.17 Nevertheless, ketamine does seem to influence evoked potentials since changes in latencies have been noted at different levels of ketamine anesthesia.17 Thus, controlling the level of ketamine/xylazine anesthesia may help reduce variability in MEPs and SEPs thereby increasing their diagnostic strength. Unfortunately, little has been done to try to define the optimal plane of anesthesia for the different types of evoked potentials in the laboratory rat.
The aim of the present study is, therefore, to determine the optimal plane of anesthesia for SEPs, BMEPs and CMEPs. We also wanted to determine whether the large differences in BMEP amplitudes that have been reported in the literature are primarily due to the plane of anesthesia. To this end, we recorded evoked potentials in rats under different levels of ketamine/xylazine anesthesia.
Methods
A total of 20 male Sprague-Dawley rats (300–350 g) were used. Rats were subjected to two different experimental protocols. In study 1 (n=8), SEPs, CMEPs and BMEPs were recorded during gradual recovery from anesthesia. In study 2 (n=12), the effect of different doses of anesthesia on BMEP morphology was studied in more detail. In both studies, animals were implanted with cranial screws for electrophysiological recording. The surgical procedures and the electrophysiological methods have been reported in detail elsewhere.8 All experimental protocols were approved by the Department for Legal Affairs of the City of Vienna and were carried out according to the Declaration of Helsinki.
Study 1
For the implantation of the cranial screws, animals were anesthetized with intraperitoneal (i.p.) injections of ketamine (75 mg/kg), xylazine (10 mg/kg), and atropine (0.3 mg/kg). Deep anesthesia was maintained with additional injections if deemed necessary. Body core temperature was kept at 37–38°C with a warming pad until recovery. Under aseptic conditions, the cranium was exposed and two stainless steel screws (4 mm length; 1.4 mm outer diameter) were implanted at the following coordinates: 2.5 mm right of midline and 3 mm posterior to bregma (coronal screw), and 1–2 mm posterior to lambda (posterior screw). The coronal screw was thus positioned over the hind limb area of the right sensorimotor cortex22 while the posterior screw was positioned just rostral of the cerebellum. The skin was sutured and the animals were allowed to recover.
One week following implantation, the animals were re-anesthetized using the same anesthetic regime as described above, and the cranial screws were exposed. No supplemental anesthetic agent was given during recording to allow gradual recovery from anesthesia. CMEPs and BMEPs were recorded in four animals (`MEP'), while SEPs were recorded in the other four rats (`SEP'). Recording was carried out every 15 min until animals had reached a light state of anesthesia. In all eight rats, the level of anesthesia was assessed according to a semi-quantitative scale at every recording time point immediately before recording. This scale is based on the degree of the hind limb withdrawal reflex (HWR) after pinch stimulation of the foot (Table 1). Additionally, the absence or presence of other reflexes (front limb withdrawal, corneal) was noted. Animals were euthanized at the end of the experiment.
Study 2
Rats (n=12) were implanted with a posterior screw only. Otherwise, anesthesia and surgery were all described for study 1. One week after implantation, animals were re-anesthetized with i.p. injections of either 100 mg/kg ketamine and 10 mg/kg xylazine (high dose (HD), n=6) or 65 mg/ml ketamine and 7 mg/ml xylazine (low dose (LD), n=6). No supplemental anesthetic agent was given during the recording period. Body core temperature was maintained at 37–38°C throughout the experiment with a warming pad. The cranial screw was re-exposed, and BMEPs were recorded 30 min after induction of anesthesia in both groups and again after 75 min in rats in the LD group. Animals were euthanized at the end of the experiment.
Electrophysiological measurements
Stimulation was carried out with a Neuromax (Excel Tech, Toronto, Canada). During stimulation, a ground needle electrode was placed subdermally over the dorsum of the neck. Signals were filtered at a bandpass frequency between 10 and 3000 Hz and stored on a computer for later analysis. Stimuli were delivered at 30 s intervals to avoid signal deterioration.7,12,22 At least two signals were recorded to ensure reproducibility.
Somatosensory evoked potentials
For SEP recording, the left hind paw was stimulated via surface electrodes positioned around the ankle and the middle of the paw. Stimuli consisted of a 100 μs impulses delivered at 2.7 Hz. Signals were recorded at supramaximal stimulation intensities (2–3 mA) from the coronal screw referenced to the posterior screw. Each signal represents the average of 60 repetitions. SEPs were evaluated by measuring peak latency and baseline-to-peak amplitude of the first negative deflection.8 Peak latency was defined as the time from the onset of the stimulation artifact to the peak of deflection.
Motor evoked potentials
BMEPs and CMEPs were both recorded via monopolar needles inserted into the belly of the tibialis anterior muscle (TA) of the left hind limb, with the reference electrode inserted into the footpad.
For BMEPs, single 100 μs impulses were applied via the posterior screw referenced to a needle electrode in the soft palate. Signals were recorded at supramaximal stimulation intensity (Imax; 10–24 mA). The evaluation of BMEPs depended on the general shape of the signals. In case a single dominant peak constituted the signal, peak latency and baseline-to-peak amplitudes were recorded. If the signal comprised several consecutive peaks, latency was again defined by the first peak, but the amplitude was defined as the largest distance between two consecutive peaks (Amax).
CMEPs were elicited by short trains of low intensity impulses (100 μs; 5–12 mA; 15.1 Hz; max. 14×) applied via the cortical screw referenced to the posterior screw.8 CMEPs were recorded at 10% above threshold intensity. Stimulation was stopped when contraction of the contralateral hind limb or a clear signal was noted. CMEP latency was defined as first-peak latency, while amplitude was Amax. CMEP duration represented the time between the onset of the first peak and the end of the last peak.
Parameters and statistical evaluation
All values are reported as mean±SD if not noted otherwise. A one-way repeated measures ANOVA was used to test whether signal parameters changed with time (study 1). In case a significant difference was detected, post hoc analysis was carried out using a Student–Newman–Keuls t-test. Regression analysis was performed with the semi-quantitative score as independent variable and the signal parameter as dependent variable. Results from study 2 were tested using one-way ANOVA followed by a Student–Newman–Keuls t-test. Significance was set at P<0.05.
Results
Study 1
Ketamine/xylazine resulted in deep anesthesia judged by the absence or near-absence of the HWR and the absence of other reflexes. Deep anesthesia lasted for approximately 45 min with HWR scores ranging between 1 and 3. The animals then started to recover from anesthesia as indicated by a gradual return of the HWR (Figure 1). At the terminal recording time point, all rats but one had reached a light stage of anesthesia judged by the presence of withdrawal and corneal reflexes. The experiments were terminated when the animals were deemed to be too light for further recordings. The SEP group showed faster recovery from anesthesia than the MEP group and therefore, no recordings were done at the 105 min timepoint in the SEP group.
Somatosensory evoked potentials
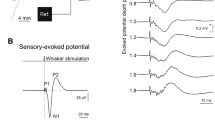
SEPs consisted of three consecutive peaks (Figure 2A). This characteristic appearance was observed at all recording time points. Since previous studies have shown that the first negative deflection (N1) is the most consistent peak,8 only the N1 was quantitatively evaluated.
Electrophysiological recordings (study 1). Representative examples of SEPs, BMEPs and CMEPs; please note that in this and all following examples the numbers in the upper left and right corners indicate the spatial (μV or mV) and the temporal resolution (ms) of one division, respectively; the arrow marks the stimulation artifact; horizontal cursors indicate peaks while vertical cursors indicate latencies (SEPs, BMEPs) or signal duration (CMEPs); the intensities (mA) used to stimulate each trace are given: (A) cortical SEPs recorded 45 min after induction of anesthesia; (B) BMEPs recorded 15 min after induction of anesthesia; note the gradual increase and the consistency of the N1; (C) BMEPs recorded 90 min after induction of anesthesia; note that in the upper trace the N1 (arrowhead) is partially obscured by the consecutive peak; (D) CMEPs recorded 105 min after induction of anesthesia
N1 latencies decreased slightly but significantly with time (15 min: 16.7±0.3 ms; 90 min: 15.4±1.6 ms; ANOVA: P<0.05). Due to this very subtle decrease, N1 latencies correlated only weakly with anesthesia levels (R=0.5; P<0.05; linear regression analysis). In contrast, N1 amplitudes were stable during deep anesthesia, but then declined rapidly to 50% of their initial value (Figure 3, ANOVA: P<0.001). This decline was also accompanied by an increase in variability. N1 amplitudes correlated strongly with the plane of anesthesia (R=0.77; P<0.001; polynomial regression analysis).
Brainstem-derived MEPs
BMEP appearance was strongly affected by the level of anesthesia. During deep anesthesia, signals featured a dominant and highly reproducible negative peak (N1), which was frequently followed by a few inconspicuous and inconsistent peaks (Figure 2B). During the following transition to light anesthesia, these consecutive peaks gradually increased in size until they dominated the signal and, in a few instances, even partially obscured the N1. However, these peaks were very inconsistent in their number and shape (Figure 2C). Therefore, evaluation was restricted to the N1.
Changing anesthesia levels greatly influenced N1 parameters. N1 latencies declined slightly but significantly (15 min: 6.7±0.1 ms; 105 min: 5.8±0.5 ms; ANOVA: P<0.005) whereas N1 amplitudes increased dramatically with time and doubled on average over the 105 min recording period (Figure 4; ANOVA, P<0.005). Variability in mean amplitude was also markedly higher during light anesthesia. Latencies and amplitudes correlated significantly with anesthesia levels (N1 latencies: R=0.62; P<0.001; N1 amplitudes: R=0.73; P<0.001; linear regression analysis). Supramaximal stimulation intensities decreased significantly with time (ANOVA, P<0.001; data not shown) and showed a very high correlation with anesthesia levels (R=0.95, P<0.001; linear regression analysis).
Corticomotor evoked potentials
During deep anesthesia, CMEPs proved to be difficult or even impossible to record. Those CMEPs that were recorded showed little reproducibility and consisted either of single waves or a few consecutive peaks. During moderate and light anesthesia, however, CMEPs were readily obtained and showed their characteristic polyphasic configuration (Figure 2D; for review see8,23). This is reflected by a significant increase in CMEP duration (Figure 5; ANOVA, P<0.001). Accordingly, CMEP duration correlated strongly with the plane of anesthesia (R=0.82; P<0.001; linear regression analysis). CMEP amplitudes and latencies, on the other hand, did not change significantly over time and also did not correlate with anesthesia levels. Mean latencies and amplitudes ranged between 18 and 21 ms and 20 and 35 μV, respectively. CMEP stimulation intensities declined significantly with time (ANOVA, P<0.001; data not shown) and correlated strongly with anesthesia levels (R=0.91; P<0.001; linear regression analysis).
Study 2
The two different doses of anesthesia resulted in distinctly different levels of anesthesia. While the withdrawal and the corneal reflexes were initially suppressed in the HD group, they were preserved in the LD group. Thus, the initial recording (30 min) was carried out under deep anesthesia in the HD group and under light anesthesia in the LD group. The second recording (75 min) in the HD group however, was done under light anesthesia when the reflexes had already returned. This is also supported by the significantly lower Imax at the 75 min recording time point (Table 2). Since Imax(HD 75 min) was also significantly lower than Imax(LD), the animals may have been lighter even than the rats in the LD group.
The different levels of anesthesia had a marked influence on BMEP appearance. The BMEPs that were recorded under deep anesthesia featured a prominent and highly reproducible N1 that was sometimes followed by several small peaks of variable latency and amplitude (Table 2, Figure 6A). BMEPs that were recorded under light anesthesia were distinctly different. They consisted either of short polyphasic signals or a single wave (Figure 6B,C). It was sometimes apparent that these peaks represented the peaks that followed the N1 (Figure 6B) while in other cases the N1 became indistinguishable due to the small spatial resolution that was necessary in order to view the whole signal (Figure 6C). In either case, these peaks represented the dominant peaks and had significantly larger amplitudes than the N1 (Table 2). Most notable, however, the variability of the latencies and amplitudes of the dominant peaks of BMEPs recorded under light anesthesia was considerably increased over that of the N1 (Figure 7, Table 2).
Electrophysiological recording (study 2). Representative examples of BMEP recordings under different anesthesia conditions; please note the different spatial resolutions for each recording; stimulus artifact in (B) and (C) not visible due to small spatial resolution; (A) BMEPs recorded 30 min after induction of deep anesthesia; (B) BMEPs recorded 75 min after induction of deep anesthesia; arrowhead: N1; (C) BMEPs recorded 30 min after induction of light anesthesia; note inconsistency in shape and amplitude; N1 probably not visible due to small resolution
Anesthesia-dependent variability. Coefficient of variation (CV) for BMEP amplitudes (study 2); open bars represent the mean (+1 SD) of the CVs of individual rats within a group (intra-animal variability); hatched bars represent the CVs of the group mean (inter-animal variability); asterisk: P<0.05 vs deep 30′
Discussion
The results of the present study clearly show that varying levels of anesthesia induced by ketamine/xylazine affect evoked potentials in the rat. All three types of evoked potentials recorded herein showed significant changes in at least one of the evaluated parameters. These changes also correlated significantly with hind limb reflex scores, which were used to indicate the plane of anesthesia. Our data also indicate that variability depends to a large degree on the plane of anesthesia.
Based on these results, it should be possible to define optimal ranges of anesthetic planes for the different evoked potentials. It seems logical that the main criterion used to define such ranges should be reproducibility within and between animals in order to obtain values with low variability. Based on the assumption and on the data of the present study, we believe that SEPs are best recorded in deeply anesthetized rats, BMEPs during moderately deep anesthesia, while CMEPs should be recorded in lightly anesthetized rats.
Our data also show that the plane of anesthesia induced by ketamine/xylazine affects the general appearance of the three evoked potentials. However, while MEPs and SEPs are both equally depressed with increasing doses of volatile and barbiturate anesthetics,15,17,24 ketamine-xylazine anesthesia does not influence the different evoked potentials in a uniform way. Thus, SEPs were depressed during light anesthesia while BMEP amplitudes were smallest during deep anesthesia. We cannot explain why ketamine-xylazine anesthesia affects different signals in different ways. However, this was not the scope of the present manuscript.
The biggest influence of the plane of anesthesia on the shape of the signals was observed for BMEPs. While BMEPs consisted mainly of a single negative peak through deep to moderately deep anesthesia, several additional peaks became apparent and became so dominant that they tended to obscure the N1 during light anesthesia. This was especially apparent when the spatial resolution of the recording window was reduced in order to be able to visualize the complete signal. This frequently resulted in the N1 becoming practically obliterated, since its amplitudes were much smaller than those of the consecutive peaks. In such instances, the N1 is not readily identifiable as an individual peak due to its insignificant size at low spatial resolution. We believe that this may have happened to those groups that reported MEPs, which were characterized by very high mean amplitudes and high variability. One could argue that the peaks consecutive to the N1 represent the actual evoked potential since their size and shape are both similar to typical electromyographic recordings obtained by peripheral stimulation. However, we believe rather that they represent artifacts. This is supported by the observation by Nashmi et al 9 and Gruner et al7 who were able to record electromyographic signals after brain stimulation in rats with completely transected spinal cords. It seems likely that the large peaks that we and others have recorded represent motion artifacts that occur when the animals are in light anesthesia but are suppressed or absent in deep anesthesia.
In the present study, the hind limb withdrawal reflex (HWR) was used as an indicator of anesthetic depth. Some authors have pointed out the HWR actually reflects the analgesic state since it is an indicator of nociception and that it is not tightly linked to anesthesia.19,25 Our results, however, indicate that nociception may be better linked to the general state of anesthesia than assumed. First, the HWR showed a very smooth increase in strength as the animals gradually recovered from anesthesia. Second, the HWR scores correlated strongly with several parameters of the recorded potentials. Since several studies have shown a strong correlation of anesthetic depth and evoked potentials.13,24 this would indicate that HWR scores quite accurately reflected anesthetic status. Moreover, the fact that different degrees in the strength of the HWR can be distinguished allowed us to differentiate between several different grades of anesthesia. Other commonly used indicators of anesthetic depth such as the corneal or the swallow reflex, would not have allowed us to do so since these reflexes are either absent or present.
In conclusion, our data strongly suggest that while ketamine/xylazine anesthesia does not affect evoked potentials to such a degree as other anesthetics, it should still be tightly controlled during electrophysiological recording in order to increase reproducibility and thereby increase the prognostic and diagnostic value of the recorded potentials. BMEPs in particular require thorough evaluation since the possibility of motion artifacts cannot be ruled out.
References
Chistyakov AV et al. Dissociation of somatosensory and motor evoked potentials in non-comatose patients after head injury. Clin Neurophysiol 1999; 110: 1080–1089.
Zhou HH & Kelly PJ . Transcranial electrical motor evoked potential monitoring for brain tumor resection. Neurosurgery 2001; 48: 1075–1080.
Asazuma T et al. Ependymomas of the spinal cord and cauda equina: an analysis of 26 cases and a review of the literature. Spinal Cord 1999; 37: 753–759.
Nagle KJ et al. Intraoperative monitoring of motor evoked potentials: a review of 116 cases. Neurology 1996; 47: 999–1004.
Baskin DS & Simpson RK . Corticomotor and somatosensory evoked potential evaluation of acute spinal cord injury in the rat. Neurosurgery 1987; 20: 871–877.
Fehlings MG, Tator CH, Linden RD & Piper IR . Motor and somatosensory evoked potentials recorded from the rat. Electroencephalogr Clin Neurophysiol 1988; 69: 65–78.
Gruner JA, Wade CK, Menna G & Stokes BT . Myoelectric evoked potentials versus locomotor recovery in chronic spinal cord injured rats. J Neurotrauma 1993; 10: 327–347.
Schlag MG, Hopf R & Redl H . Serial recording of sensory, corticomotor, and brainstem-derived motor evoked potentials in the rat. Somatosens Mot Res 2001; 18: 106–116.
Nashmi R, Imamura H, Tator CH & Fehlings MG . Serial recording of somatosensory and myoelectric motor evoked potentials: role in assessing functional recovery after graded spinal cord injury in the rat. J Neurotrauma 1997; 14: 151–159.
Sun T et al. Brainsteam-evoked muscle potentials: their prognostic value in experimental spinal cord injury in the rat. Somatosens Mot Res 2000; 17: 325–332.
Zileli M & Schramm J . Motor versus somatosensory evoked potential changes after acute experimental spinal cord injury in rats. Acta Neurochir (Wien) 1991; 108: 140–147.
Gruner JA & Yee AK . 4-Aminopyridine enhances motor evoked potentials following graded spinal cord compression injury in rats. Brain Res 1999; 816: 446–456.
Yamada H et al. The effects of volatile anesthetics on the relative amplitudes and latencies of spinal and muscle potentials evoked by transcranial magnetic stimulation. Spine 1994; 19: 1512–1517.
Russell GB, Schwentker MC & Graybeal JM . Preservation of neurogenic motor-evoked potentials during isoflurane electroencephalographic burst suppression in rats. Spine 1994; 19: 2632–2636.
Chiba A et al. Magnetically induced motor evoked potentials and H-reflex during nembutal and ketamine anesthesia administration in rats. Res Commun Mol Pathol Pharmacol 1998; 101: 43–57.
Koyanagi I & Tator CH . The effects of cortical stimulation, anesthesia and recording site on somatosensory evoked potentials in the rat. Electroencephalogr Clin Neurophysiol 1996; 101: 534–542.
Goss-Sampson MA & Kriss A . Effects of pentobarbital and ketamine-xylazine anaesthesia on somatosensory, brainstem auditory and peripheral sensory-motor responses in the rat. Lab Anim 1991; 25: 360–366.
Zhou HH & Zhu C . Comparison of isoflurane effects on motor evoked potential and F wave. Anesthesiology 2000; 93: 32–38.
Wixson SK et al. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on noxious stimulus perception in adult male rats. Lab Anim Sci 1987; 37: 731–735.
Wixson SK et al. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam anesthesia in adult male rats. Lab Anim Sci 1987; 37: 726–730.
Wixson SK et al. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on arterial blood pH, blood gases, mean arterial blood pressure and heart rate in adult male rats. Lab Anim Sci 1987; 37: 736–742.
Hall RD & Lindholm EP . Organization of motor and somatosensory neocortex in the albino rat. Brain Res 1974; 66: 23–38.
Kalderon N & Fuks Z . Severed corticospinal axons recover electrophysiologic control of muscle activity after x-ray therapy in lesioned adult spinal cord. Proc Natl Acad Sci USA 1996; 93: 11185–11190.
Zentner J, Albrecht T & Heuser D . Influence of halothane, enflurane, and isoflurane on motor evoked potentials. Neurosurgery 1992; 31: 298–305.
Haberham ZL et al. EEG evaluation of reflex testing as assessment of depth of pentobarbital anaesthesia in the rat. Lab Amin 1999; 33: 47–57.
Acknowledgements
We thank Nurit Kalderon, Sloan Kettering Institute, New York, for helpful discussion.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zandieh, S., Hopf, R., Redl, H. et al. The effect of ketamine/xylazine anesthesia on sensory and motor evoked potentials in the rat. Spinal Cord 41, 16–22 (2003). https://doi.org/10.1038/sj.sc.3101400
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101400
Keywords
This article is cited by
-
Focused ultrasound enhances the anesthetic effects of topical lidocaine in rats
BMC Anesthesiology (2021)
-
Low-pressure micro-mechanical re-adaptation device sustainably and effectively improves locomotor recovery from complete spinal cord injury
Communications Biology (2018)
-
Characterizing Brain Cortical Plasticity and Network Dynamics Across the Age-Span in Health and Disease with TMS-EEG and TMS-fMRI
Brain Topography (2011)
-
Dipolar cortico-muscular electrical stimulation: a novel method that enhances motor function in both - normal and spinal cord injured mice
Journal of NeuroEngineering and Rehabilitation (2010)
-
NON-activated protein C as post-treatment after spinal cord compression injury in rats
Acta Neurochirurgica (2006)