Abstract
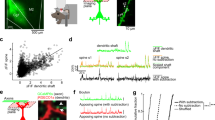
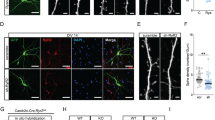
The NMDA (N -methyl-D-aspartate) subclass of glutamate receptor1 is essential for the synaptic plasticity thought to underlie learning and memory2,3,4 and for synaptic refinement during development5,6. It is currently believed that the NMDA receptor (NMDAR) is a heteromultimeric channel comprising the ubiquitous NR1 subunit and at least one regionally localized NR2 subunit7,8,9,10,11. Here we report the characterization of a regulatory NMDAR subunit, NR3A (formerly termed NMDAR-L or χ-1), which is expressed primarily during brain development12,13. NR3Aco-immunoprecipitates with receptor subunits NR1 and NR2 in cerebrocortical extracts. In single-channel recordings from Xenopus oocytes, addition of NR3A to NR1 and NR2 leads to the appearance of a smaller unitary conductance. Genetic knockout of NR3A in mice results in enhanced NMDA responses and increased dendritic spines in early postnatal cerebrocortical neurons. These data suggest that NR3A is involved in the development of synaptic elements by modulating NMDAR activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McBain, C. J. & Mayer, M. L. N -methyl-D-aspartic acid receptor structure and function. Physiol. Rev. 74, 723–760 (1994).
Gustafsson, B. & Wigstrom, H. Physiological mechanisms underlying long-term potentiation. Trends Neurosci. 11, 156–162 (1988).
Madison, D. V., Malenka, R. C. & Nicoll, R. A. Mechanisms underlying long-term potentiation of synaptic transmission. Annu. Rev. Neurosci. 14, 379–397 (1991).
Bliss, T. V. P. & Collingridge, G. L. Asynaptic model of memory: long term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Constantine-Paton, M., Cline, H. T. & Debski, E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu. Rev. Neurosci. 13, 129–154 (1990).
Singer, W. Development and plasticity of cortical processing architectures. Science 270, 758–764 (1995).
Moriyoshi, K. et al. Molecular cloning and characterization of the rat NMDA receptor. Nature 354, 31–37 (1991).
Kutsuwada, T. et al. Molecular diversity of the NMDA receptor channel. Nature 358, 36–41 (1992).
Monyer, H. et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256, 1217–1221 (1992).
Sheng, M., Cummings, J., Roldan, L. A., Jan, Y. N. & Jan, L. Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368, 144–147 (1994).
Hollmann, M. & Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108 (1994).
Sucher, N. J. et al. Development and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J. Neurosci. 15, 6509–6520 (1995).
Ciabarra, A. M. et al. Cloning and characterization of χ;-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J. Neurosci. 15, 6498–6508 (1995).
Stern, P., Miroslav, C., Colquhoun, D. & Stephenson, F. A. Single channel properties of cloned NMDA receptors in a human cell line: comparison with results from Xenopus oocytes. J. Physiol. (Lond.) 476, 391–397 (1994).
Premkumar, L. S. & Auerbach, A. Stoichiometry of recombinant N -methyl-D-aspartate receptor channels inferred from single-channel current patterns. J. Gen. Physiol. 110, 485–502 (1997).
Monyer, H., Burnashev, N., Laurie, D. J., Sakmann, B. & Seeburg, P. H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540 (1994).
Lambolez, B., Audinat, E., Bochet, P., Crepel, F. & Rossier, J. AMPA receptor subunits expressed by single Purkinje cells. Neuron 9, 247–258 (1992).
McAllister, A. K., Katz, L. C. & Lo, D. C. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 17, 1057–1064 (1996).
Woolley, C. S. & McEwen, B. S. Estradiol regulates hippocampal dendritic spine density via an N -methyl-D-aspartate receptor-dependent mechanism. J. Neurosci. 14, 7680–7687 (1994).
Woolley, C. S., Weiland, N. G., McEwen, B. S. & Schwartzkroin, P. A. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci. 17, 1848–1859 (1997).
1. Harris, K. M. & Kater, S. B. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 17, 341–371 (1994).
Segal, M. Dendritic spines for neuroprotection: a hypothesis. Trends Neurosci. 18, 468–471 (1995).
Papa, M. & Segal, M. Morphological plasticity in dendritic spines of cultured hippocampal neurons. Neuroscience 71, 1005–1011 (1996).
Kossel, A., Williams, C. V., Scheizer, M. & Kater, S. B. Afferent innervation influences the development of dendritic branches and spines via both activity-dependent and non-activity-dependent mechanisms. J. Neurosci. 17, 6314–6324 (1997).
Rogers, S. W. et al. The characterization and localization of the glutamate receptor subunit GluR1 in the rat brain. J. Neurosci. 11, 2713–2724 (1991).
Hollmann, M. et al. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron 10, 943–954 (1993).
Chen, H.-S. V. et al. Open-channel block of NMDA responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 12, 4427–4436 (1992).
Chen, H.-S. V. & Lipton, S. A. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J. Physiol. (Lond.) 499, 27–46 (1997).
Sucher, N. J., Awobuluyi, M., Choi, Y.-B. & Lipton, S. A. NMDA receptors: from genes to channels. Trends Pharmacol. Sci. 17, 348–355 (1996).
Acknowledgements
We thank N. J. Sucher, M. Wyszynski, D. Zurakowski, J. Yuan, T. S. Khurana, C.Yiannoutsos and K. Rosen for advice and discussion; N. J. Sucher, M. Sheng, R. Wenthold and K.Buckley for antibodies; and J. Cohen and M. Sheng for critical reading of the manuscript. This work was supported by fellowships from the HHMI (S.D., W.C.) and the Harvard Mahoney Neuroscience Institute (Y.F.S.), grants from the NIH (J.E.C., S.A.L., N.N.), the Klingenstein Foundation, the Edward R. and Anne G. Lefler Center, NARSAD and the Funds for Discovery (N.N.). S.A.L. has consultancy and sponsored research agreements with Neurobiological Technologies, Inc. (Richmond, CA) and Allergan, Inc. (Irvine, CA) in the field of NMDAR antagonists.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Das, S., Sasaki, Y., Rothe, T. et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393, 377–381 (1998). https://doi.org/10.1038/30748
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/30748
This article is cited by
-
DNA methylation and the opposing NMDAR dysfunction in schizophrenia and major depression disorders: a converging model for the therapeutic effects of psychedelic compounds in the treatment of psychiatric illness
Molecular Psychiatry (2023)
-
Progresses in GluN2A-containing NMDA Receptors and their Selective Regulators
Cellular and Molecular Neurobiology (2023)
-
Aß Pathology and Neuron–Glia Interactions: A Synaptocentric View
Neurochemical Research (2023)
-
A perspective on molecular signalling dysfunction, its clinical relevance and therapeutics in autism spectrum disorder
Experimental Brain Research (2022)
-
Evaluation of spinal cord protective threshold of serum memantine, an NMDA receptor antagonist, in a rabbit model of paraplegia
Indian Journal of Thoracic and Cardiovascular Surgery (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.