Abstract
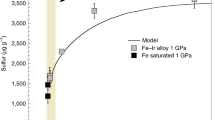
The solubility of rare gases in silicate melts and minerals at high pressure is of importance for understanding the early history of the Earth and its present day degassing. Helium, neon, argon, krypton and xenon were originally incorporated into the Earth during its accretion, and have also been produced by radioactive decay1. These elements have been used as tracers for deciphering mantle structure and constraining the number and size of geochemical reservoirs1,2,3. In particular, it has been proposed that the budget of 40Ar, produced by the radioactive decay of 40K, provides the strongest argument for chemical layering within the mantle1,4. The geochemical models used to arrive at this conclusion are, however, currently under re-examination5, with a large source of uncertainty being the lack of data on argon partitioning during melting. It has previously been assumed, on the basis of low-pressure data, that noble gases are highly soluble in melts at all pressures. But here we present solubility data of argon in olivine melt at very high pressure that indicate that argon solubility is strongly dependent on pressure, especially in the range of 4–5 gigapascals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jambon, A. in Volatiles in Magmas (eds Carroll, M. R. and Holloway, J. R.) 479–517 (Mineralogical Soc. Am., Washington DC, (1994)).
Zhang, Y. & Zindler, A. Noble gas constraints on the evolution of the Earth's atmosphere. J. Geophys. Res. 94, 13719–13737 (1989).
Azbel, I. & Tolstikhin, I. N. Geodynamics, magmatism, and degassing of the Earth. Geochim. Cosmochim. Acta 54, 139–154 (1990).
Allègre, C., Hofmann, A. & O'Nions, K. The argon constraints on mantle structure. Geophys. Res. Lett. 23, 3555–3557 (1996).
Albarède, F. Time-dependent models of U–Th–He and K–Ar evolution and the layering of mantle convection. Chem. Geol. (in the press).
White, B. S., Brearley, M. & Montana, A. Solubility of argon in silicate liquids at high pressures. Am. Mineral. 74, 513–5229 (1989).
Montana, A., Guo, Q., Boettcher, S., White, B. S. & Brearley, M. Xe and Ar in high pressure silicate liquids. Am. Mineral. 78, 1135–1142 (1993).
Carroll, M. L. & Stolper, E. M. Noble gas solubilities in silicate melts and glasses: new experimental results for argon and the relationships between solubility and ionic porosity. Geochim. Cosmochim. Acta 75, 5039–5051 (1993).
Chamorro-Perez, E., Gillet, P. & Jambon, A. Argon solubility in silicate melts at very high pressures. Experimental set-up and preliminary results for anorthite and silica melts. Earth Planet. Sci. Lett. 145, 97–107 (1996).
Wang, S. Y., Sharma, S. K. & Cooney, T. F. Micro-Raman and infrared spectral study of forsterite under high pressure. Am. Mineral. 78, 469–476 (1993).
Gillet, P., Fiquet, G., Daniel, I. & Reynard, B. Raman spectroscopy at mantle pressure and temperature conditions. Experimental set-up and the example of CaTiO3perovskite. Geophys. Res. Lett. 20, 1931–1934 (1993).
Ohtani, E. & Kuazawa, M. Melting of forsterite Mg2SiO4up to 15 GPa. Phys. Earth Planet. Inter. 27, 32–38 (1981).
Shackelford, J. & Masaryk, J. The interstitial structure of vitreous silica. J. Non-Cryst. Solids 30, 127–139 (1978).
Shibata, T., Takahashi, E. & Matsuda, J. Noble gas solubility in binary CaO–SiO2system. Geophys. Res. Lett. 23, 3139–3142 (1996).
Agee, C. & Walker, D. Static compression and olivine flotation in ultrabasic silicate liquid. J. Geophys. Res. 93, 3437–3449 (1988).
Poole, P. H., McMillan, P. F. & Wolf, G. H. in Structure, Dynamics and Properties of Silicate Melts (eds Stebbins, J. F., McMillan, P. F. and Dingwell, D. B.) 563–616 (Mineralogical Soc. Am., Washington DC, (1995)).
Brooker, A., Wartho, J.-A., Carroll, M. & Kelley, P. Preliminary UVLAMP determinations of argon partition coefficients for olivine and clinopyroxene grown from silicate melts. Chem. Geol. (submitted).
Acknowledgements
We thank P. Blanc for his assistance with the SEM, D. Badia for help with microprobe analysis and K. Roselieb for giving the Ar–SiO2 standard. We are grateful to R. A. Brooker for making his manuscript available before publication. This work was financially supported by the INSU program ‘DBT: Terre Profonde’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chamorro-Pérez, E., Gillet, P., Jambon, A. et al. Low argon solubility in silicate melts at high pressure. Nature 393, 352–355 (1998). https://doi.org/10.1038/30706
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/30706
This article is cited by
-
Noble gas transport into the mantle facilitated by high solubility in amphibole
Nature Geoscience (2013)
-
Primary and secondary processes constraining the noble gas isotopic signatures of carbonatites and silicate rocks from Brava Island: evidence for a lower mantle origin of the Cape Verde plume
Contributions to Mineralogy and Petrology (2012)
-
Helium penetrates into silica glass and reduces its compressibility
Nature Communications (2011)
-
Aluminium control of argon solubility in silicate melts under pressure
Nature (2006)
-
Breaking of Henry's law for noble gas and CO2 solubility in silicate melt under pressure
Nature (2005)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.