Abstract
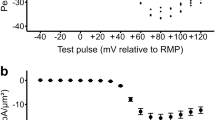
The sensitivities of the cardiac (Na+ + K+)ATPases to inhibition by ouabain have been shown to correlate with the pharmacologically active digitalis concentrations in the various source species1. The rat heart enzyme is generally considered to be insensitive to ouabain with half-maximal inhibition at ∼6 × 10−5 M ouabain2,3 and a KD for binding3–7 of 1–6 × 10−5 M. However, recent data5–7 showed that rat cardiac sarcolemma preparations can bind ouabain with high affinity (KD 1–3 × 10−7M) with no detectable concomitant enzyme inhibition in vitro but producing a sustained positive inotropic effect in vivo. In the present study, we measured the (Na+ + K+)ATPase inhibition by ouabain in sarcolemma-enriched fractions obtained from rat hearts relaxed by perfusion with Ca2+-free solutions. In these conditions, two enzyme forms having high and low sensitivities to ouabain were found (half-maximal inhibitions with 1.2×10−8 and 6×10−5M, respectively). Their relative proportion is close to 1. Confirming previous data2–7, the highly sensitive form was not detected in preparations from hearts maintained at a physiological calcium level. This heterogeneous population of functional (Na+ + K+)ATPases suggests that, in rat heart, the positive inotropic effect of low doses of ouabain in vivo5–9 would act through the inhibition9 of the enzyme activity highly sensitive to ouabain as detected in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Repke, K., Est, M. & Portius, H. J. Biochem. Pharmac. 14, 1785–1805 (1965).
Wallick, E. T., Dowd, F., Allen, J. C. & Schwartz, A. J. Pharmac. exp. Ther. 189, 434–444 (1974).
Allen, J. C. & Schwartz, A. J. Pharmac. exp. Ther. 168, 42–46 (1969).
Dnansfeld, H., Greeff, H. K., Berger, H. & Cautius, V. Naumyn-Schmiedeberg's Archs Pharmak. 254, 225–237 (1966).
Erdmann, E., Philipp, G. & Scholz, H. Biochem. Pharmac. 29, 3219–3229 (1980).
Adams, R. J. et al. Nature 296, 167–169 (1982).
Finet, M., Noel, F. & Godfraind, T. Archs int. Pharmacodyn. Ther. 256, 168–170 (1982).
Godfraind, T. & Ghysel-Burton, J. Proc. natn. Acad. Sci. U.S.A. 77, 3067–3069 (1980).
Akera, T. and Brody, T. M. Pharmac. Rev. 29, 187–220 (1979).
Swynghedauw, B., Bouveret, P. & Hatt, P. Y. J. molec. cell. Cardiol. 5, 441–459 (1973).
Powell, T., Terrar, D. A. & Twist, V. W. J. Physiol., Land. 302, 131–153 (1980).
Jorgensen, P. L. & Skou, J. C. Biochim. biophys. Acta 233, 366–380 (1971).
Ku, D. D., Akera, T., Tobin, T. & Brody, T. M. J. Pharmac. exp. Ther. 197, 458–469 (1976).
Pitts, B. J. R. J. biol. Chem. 254, 6232–6235 (1979).
Frick, P. G. & Lowenstein, J. M. J. biol. Chem. 251, 6372–6378 (1976).
Zak, R., Etlinger, J. & Fischman, D. A. Excerpta Medica int. Congr. Ser. 240, 163–175 (1980).
Akera, T. Biochim. biophys. Acta 249, 52–62 (1971).
Sweadner, K. J. J. biol. Chem. 254, 6060–6067 (1979).
Cutiletta, A. F., Aumont, M. C., Nag, A. C. & Zak, R. J. molec. cell. Cardiol. 9, 399–407 (1977).
Hunter, D. R., Haworth, R. A. & Berkoff, H. A. Proc. natn. Acad. Sci. U.S.A. 78, 5665–5668 (1981).
Carrier, G. O., Lüllmann, H., Naubauer, L. & Peters, T. J. molec. cell. Cardiol. 6, 333–347 (1974).
Koomen, J. M., Van Gilst, W. H., Zimmerman, A. N. E. & Van Noordwijk, J. Archs int. Pharmacodyn. Ther. 255, 212–219 (1982).
Lelièvre, L. G., Zachowski, A., Charlemagne, D., Laget, P. & Paraf, A. Biochim. biophys. Acta 557, 399–408 (1979).
Lelièvre, L. G., Piascik, M. T., Potter, J. D., Wallick, E. T. & Schwartz, A. in Proc. 3rd int. Na, K-ATPase Conf. (Academic, New York, in the press).
Geny, B., Paraf, A., Fedon, Y. & Charlemagne, D. Biochim. biophys. Acta (in the press).
Noble, D. Cardiovasc. Res. 14, 495–514 (1980).
Lowry, O. H., Rosenbrough, N. J., Farr, A. L. & Randall, R. J. J. biol. Chem. 193, 265–275 (1951).
Anner, B. & Moosmayer, M. Analyt. Biochem. 65, 305–309 (1975).
Evans, W. H. in Laboratory Techniques in Biochemistry and Molecular Biology Vol. 7, Pt 1 (eds Work, T. S. & Work, E.) (North-Holland, Amsterdam, 1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mansier, P., Lelievre, L. Ca2+-free perfusion of rat heart reveals a (Na+ + K+) ATPase form highly sensitive to ouabain. Nature 300, 535–537 (1982). https://doi.org/10.1038/300535a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/300535a0
This article is cited by
-
Influence of digoxin on the Na,K-ATPase activity, transmembrane potential, and contractile activity of ischemically damaged rat heart
Pharmaceutical Chemistry Journal (1992)
-
Inotropic effect of ouabain in hypertrophied rat heart
Pfl�gers Archiv European Journal of Physiology (1990)
-
Diminished toxicity of ouabain in the hypertrophied rat heart
Pfl�gers Archiv European Journal of Physiology (1989)
-
Postnatal changes in the inotropic effect of ouabain on the rat heart ventricle
Basic Research in Cardiology (1987)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.