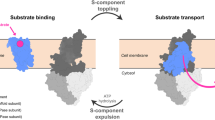
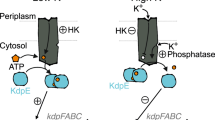
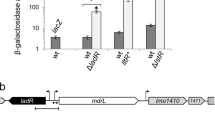
Abstract
The nucleotide sequence of the entire histidine transport operon from Salmonella typhimurium has been determined and is shown to consist of four genes, hisJ, hisQ, hisM and hisP. This operon provides the only example of a binding protein-dependent transport system for which the total number of protein components is known. Determination of the amino acid compositions and sequences of these four transport proteins, together with analysis of various transport mutants, allows us to propose a molecular model for binding protein-dependent transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ames, G. F.-L. in 1st ICN-UCLA Symp. molec. Biol. (ed. Fox, C. F.) 409–426 (Academic, New York, 1972).
Oxender, D. L. & Quay, S. C. in Methods of Membrane Biology Vol. 6 (ed. Korn E. D.) 183–242 (Plenum, New York, 1976).
Buchel, D. E., Gronenborn, B. & Muller-Hill, B. Nature 283, 541–545 (1980).
Ames, G. F.-L. et al. J. Bact. 129, 1289–1297 (1977).
Ames, G. F.-L. & Lever, J. E. J. biol. Chem. 247, 4309–4316 (1972).
Ames, G. F.-L. & Nikaido, K. Proc. natn. Acad. Sci. U.S.A. 75, 5447–5451 (1978).
Ames, G. F.-L. & Spudich, E. N. Proc. natn. Acad. Sci. U.S.A. 73, 1887–1881 (1976).
Kustu, S. G. & Ames, G. F.-L. J. Bact. 166, 107–113 (1973).
Kustu, S. G., McFarland, N. C., Hui, S. P., Esmond, B. & Ames, G. F.-L. J. Bact. 138, 218–234 (1979).
Higgins, C. F. & Ames, G. F.-L. Proc. natn. Acad. Sci. U.S.A. 78, 6038–6042 (1981).
Higgins, C. F. & Ames, G. F.-L. Proc. natn. Acad. Sci. U.S.A. 79, 1083–1087 (1982).
Ardeshir, F. & Ames, G. F.-L. J. supramolec. Struct. 13, 117–130 (1980).
Ardeshir, F., Higgins, C. F. & Ames, G. F.-L. J. Bact. 147, 401–409 (1981).
Hogg, R. W. J. biol. Chem. 256, 1935–1939 (1981).
Noel, D., Nikaido, K. & Ames, G. F.-L. Biochemistry 18, 4159–4165 (1979).
O'Farrell, P. Z., Goodman, H. M. & O'Farrell, P. H. Cell 12, 1133–1142 (1977).
Jaskunas, S. R., Lindahl, L. & Nomura, M. Proc. natn. Acad. Sci. U.S.A. 72, 6–10 (1975).
Young, I. G., Rogers, B. L., Campbell, H. D., Jaworowski, A. & Shaw, D. C. Eur. J. Biochem. 116, 165–170 (1981).
Borer, P. N., Dengler, B. & Tinoco, I. J. molec. Biol. 86, 845–853 (1974).
Higgins, C. F., Ames, G. F.-L., Barnes, W., Clement, J.-M. & Hofnung, M. Nature 298, 760–762 (1982).
Selker, E. & Yanofsky, C. J. molec. Biol. 130, 135–143 (1979).
Christie, G. E. & Platt, T. J. molec. Biol. 142, 519–530 (1980).
Shine, J. & Dalgarno, L. Proc. natn. Acad. Sci. U.S.A. 71, 1342–1346 (1974).
Rosenberg, M. & Court, D. A. Rev. Genet. 13, 319–353 (1979).
Emr, S. D., Hall, M. N. & Silhavy, T. J. J. Cell Biol. 86, 701–711 (1980).
Gay, N. J. & Walker, J. E. Nucleic Acids Res. 9, 2187–2194, 3919–3926 (1981).
Chang, S. M. et al. Proc. natn. Acad. Sci. U.S.A. 78, 3398–3402 (1981).
Dellwag, H. G. & Sumper, M. FEBS Lett. 116, 303–309 (1980).
Pratt, J. M., Holland, I. B. & Spratt, B. G. Nature 293, 307–309 (1981).
Ehring, R., Beyreuther, K., Wright, J. K. & Overath, P. Nature 283, 537–540 (1980).
Palmiter, R. D., Gagnon, J. & Walsh, K. A. Proc. natn. Acad. Sci. U.S.A. 75, 94–98 (1978).
O'Farrell, P. H. J. biol. Chem. 250, 4007–4021 (1975).
Segrest, J. P. & Feldman, R. J. J. molec. Biol. 87, 853–858 (1974).
Randall, L. & Schwartz, M. J. Bact. 116, 1436–1446 (1973).
Shuman, H. A., Silhavy, T. J. & Beckwith, J. R. J. biol. Chem. 255, 168–174 (1981).
Bavoil, P., Hofnung, M. & Nikaido, H. J. biol. Chem. 255, 8366–8369 (1980).
Shuman, H. A. & Silhavy, T. J. J. biol. Chem. 256, 560–562 (1981).
Silhavy, T. J. et al. Molec. gen. Genet. 174, 249–259 (1979).
Anderson, J. J. & Oxender, D. L. J. Bact. 130, 384–392 (1977).
Lo, T. C. Y. J. supramolec. Struct. 7, 463–480 (1977).
Ordal, G. W. & Adler, J. J. Bact. 117, 517–526 (1974).
Robertson, D. E., Kroon, P. A. & Ho, C. Biochemistry 16, 1443–1451 (1977).
Singer, S. J. A. Rev. Biochem. 43, 805–833 (1974).
Maxam, A. & Gilbert, W. Meth. Enzym. 65, 499–560 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Higgins, C., Haag, P., Nikaido, K. et al. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature 298, 723–727 (1982). https://doi.org/10.1038/298723a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/298723a0
This article is cited by
-
Structure and function of ABC transporters: the ATP switch provides flexible control
Pflügers Archiv - European Journal of Physiology (2007)
-
H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB
The EMBO Journal (2005)
-
The ATP switch model for ABC transporters
Nature Structural & Molecular Biology (2004)
-
Cloning and sequencing of the nitrate transport system from the thermophilic, filamentous cyanobacterium Phormidium laminosum: comparative analysis with the homologous system from Synechococcus sp. PCC 7942
Plant Molecular Biology (1995)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.