Abstract
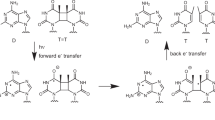
One of the principal photochemical reactions of DNA on exposure to UV is the formation of intrastrand cyclobutane-type pyrimidine dimmers1,2. The efficiency of this reaction depends on both the wavelength of the UV2 and the specific nucleotide sequence in the DNA3. The formation of the pyrimidine dimer and its repair in living cells have been studied extensively4. We have examined the possibility that pyrimidines at the ends of DNA strands may be adequately juxtaposed for dimer formation by the presence of a complementary strand, even when no phosphodiester linkage joins their sugars. In these conditions the formation of a dimer will ‘ligate’ two DNA strands end-to-end. We report here that thymidine oligonucleotides annealed to polydeoxyadenylate can be ligated end-to-end by UV irradiation, via thymine dimerization of the terminal nucleotides in adjacent oligonucleotides. The linkages are susceptible to direct photoreversal by 254 nm UV, as expected for cyclobutane-type thymine dimers, but they are not cleaved by the bacteriophage T4 endonuclease V, a dimer-specific DNA repair enzyme. We demonstrate that the ligating dimers are also resistant to photolyase from Escherichia coli. Although the phosphodiester backbone is not required for dimer formation, it is required for recognition of dimers by these DNA repair enzymes. We discuss the possibility that high molecular weight polynucleotides in primordial seas might have been generated from oligonucleotides by pyrimidine dimerization under the intense solar UV flux unattenuated by an ozone layer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Smith, K. C. & Hanawalt, P. C. Molecular Photobiology (Academic, New York, 1969).
Deering, R. A. & Setlow, R. B. Biochim. biophys. Acta 68, 526–531 (1963).
Haseltine, W. A. et al. Nature 285, 634–641 (1980).
Hanawalt, P. C., Cooper, P. K., Ganesan, A. K. & Smith, C. A. A. Rev. Biochem. 48, 783–836 (1979).
Wang, Shih Yi. Photochemistry and Photobiology of Nucleic Acids Vol. 2, 430 (Academic, New York, 1976).
Eisinger, J. et al. Proc. natn. Acad. Sci. U.S.A. 55, 1015–1020 (1966).
Rahn, R. O. & Landry, L. C. Photochem. Photobiol. 18, 29–38 (1973).
Spencer, J. H. The Physics and Chemistry of DNA and RNA, 92 (Saunders, Philadelphia, 1972).
Janik, B. Physicochemical Characteristics of Oligonucleotides and Polynucleotides, 108–109 (Plenum, New York, 1971).
Reynolds, R. J., Cook, K. H. & Friedberg, E. C. in DNA Repair: A Laboratory Manual of Research Procedures (eds Friedberg, E. C. & Hanawalt, P. C.) 11–22 (Dekker New York, 1981).
Haynes, F. N., Wilkins, D. L., Ratliff, R. L., Varghese, A. J. & Rupert, C. S. J. Am. chem. Soc. 93, 4940–4942 (1971).
Sutherland, J. C. Photochem. Photobiol. 25, 435–440 (1977).
Snapka, R. M. & Sutherland, B. M. Biochemistry 19, 4201–4208 (1980).
Setlow, J. K. & Bollem, F. J. Biochim. biophys. Acta 157, 233–237 (1967).
Freidberg, E. C. Mutat. Res. 15, 113–123 (1972).
Seawell, P. C., Smith, C. A. & Ganesan, A. K. J. Virol, 35, 790–797 (1980).
Tśo, P. O. Basic Principles in Nucleic Acid Chemistry Vol. 1 (Academic, New York, 1974).
Bridson, P. K. & Orgel, L. E. J. molec. Biol. 144, 567–577 (1980).
Ibanez, J. D., Kimball, A. P. & Oro, J. Science 173, 444–446 (1971).
Maxam, A. & Gilbert, W. Meth. Enzym. 65, 499–560 (1980).
Haselkorn, R. & Doty, P. J. biol. Chem. 236(10), 2738–2745 (1961).
Peacock, A. C. & Dingman, C. W. Biochemistry 6(b), 1818–1827 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lewis, R., Hanawalt, P. Ligation of oligonucleotides by pyrimidine dimers—a missing ‘link’ in the origin of life?. Nature 298, 393–396 (1982). https://doi.org/10.1038/298393a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/298393a0
This article is cited by
-
Molecular models of photodamaged DNA
Reviews of Chemical Intermediates (1988)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.