Abstract
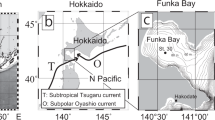
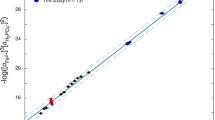
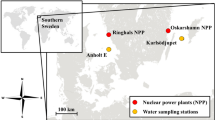
IODINE in seawater is present as iodate (IO3−) and as iodide (I−)1–3. Although there is some uncertainty in the true redox potentials of natural waters4, the thermodynamically stable species of iodine in seawater, and in river waters which are well oxygenated, is iodate5–7. We have studied an estuary to determine the distribution of iodine species as a function of salinity, and examined the results for evidence of interconversion of species in the estuary. We report here that iodide is the dominant form in river water and is oxidised to the thermodynamically stable iodate in the sea, not in the estuary.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sugawara, K. & Terada, K. Nature 258, 250–251 (1958).
Johannesson, J. K. Nature 258, 251 (1958).
Barkley, R. A. & Thompson, T. G. Deep-Sea Res. 7, 24–34 (1960).
Breck, W. G. in The Sea 5, (ed. Goldberg, E. D.) 153–179 (Wiley-Interscience, New York, 1974).
Sillen, L. G. in Oceanography (ed. Sears, M.) 549–581 (AAAS, Washington, D. C, 1961).
Stumm, W. & Brauner, P. A. in Chemical Oceanography, 2nd edn (eds Riley, J. P. & Skirrow, G.) Vol. 1, 173–239 (Academic, London, 1975).
Garrels, R. M. & Christ, C. L. Solutions, Minerals and Equilibria 381 (Harper & Row, New York, 1965).
Blutstein, H. & Smith, J. D. Water Res. 12, 119–125 (1978).
Petek, M. & Branica, M. Rapp. Comm. int. Mer Médit. 19, 767 (1969).
Herring, J. R. & Liss, P. S. Deep-Sea Res. 21, 777–783 (1974).
Whitnack, G. C. Analyt. Chem. 47, 618–621 (1975).
Fuge, R. in Handbook of Geochemistry, Vol. II-3, Sections 53 H/I (ed. Wedepohl, K. H.) (Springer, Berlin, 1974).
Truesdale, V. W. Mar. Chem. 6, 1–13 (1978).
Wong, G. T. F. Deep-Sea Res. 24, 115–125 (1977).
Sugawara, K. & Terada, K. Inf. Bull. Planktol. Jap. Commemoration No. of Dr Y. Matsue, 213–218 (1967).
Truesdale, V. W. Mar. Chem. 3, 111–119 (1975).
Brewer, P. G. in Chemical Oceanography, 2nd edn (eds Riley, J. P. & Skirrow, G.) Vol. 1, 459 (Academic, London, 1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SMITH, J., BUTLER, E. Speciation of dissolved iodine in estuarine waters. Nature 277, 468–469 (1979). https://doi.org/10.1038/277468a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/277468a0
This article is cited by
-
Liquid waveguide capillary cell for the spectrophotometric determination of nanomolar iodate concentrations in marine waters
Acta Oceanologica Sinica (2022)
-
Multiple geochemical factors may cause iodine and selenium deficiency in Gilgit-Baltistan, Pakistan
Environmental Geochemistry and Health (2021)
-
Preliminary Evidence for Iodate Reduction in Bottom Waters of the Gulf of Mexico During an Hypoxic Event
Aquatic Geochemistry (2011)
-
The geochemistry of iodine — a review
Environmental Geochemistry and Health (1986)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.