Abstract
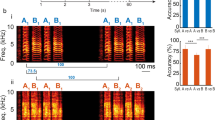
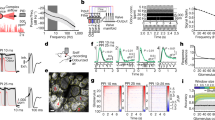
Our inferences about brain mechanisms underlying perception rely on whether it is possible for the brain to ‘reconstruct’ a stimulus from the information contained in the spike trains from many neurons1,2,3,4,5. How the brain actually accomplishes this reconstruction remains largely unknown. Oscillatory and synchronized activities in the brain of mammals have been correlated with distinct behavioural states or the execution of complex cognitive tasks6,7,8,9,10,11 and are proposed to participate in the ‘binding’ of individual features into more complex percepts12,13,14,. But if synchronization is indeed relevant, what senses it? In insects, oscillatory synchronized activity in the early olfactory system seems to be necessary for fine odour discrimination15 and enables the encoding of information about a stimulus in spike times relative to the oscillatory ‘clock’16. Here we study the decoding of these coherent oscillatory signals. We identify a population of neurons downstream from the odour-activated, synchronized neuronal assemblies. These downstream neurons show odour responses whose specificity is degraded when their inputs are desynchronized. This degradation of selectivity consists of the appearance of responses to new odours and a loss ofdiscrimination of spike trains evoked by different odours. Suchloss of information is never observed in the upstream neurons whose activity is desynchronized. These results indicate that information encoded in time across ensembles of neurons converges onto single neurons downstream in the pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mountcastle, V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex J. Neurophysiol. 20, 408–434 (1957).
Sparks, D. L., Lee, C. & Rohrer, W. H. Population coding of the direction, amplitude and velocity of saccadic eye movements by neurons in the superior colliculus Cold Spring Harb. Symp. Quant. Biol. 55, 805–811 (1990).
Georgopoulos, A. P., Lurito, J. T., Petrides, M., Schwartz, A. B. & Massey, J. T. Mental rotation of the neuronal population vector Science 243, 234–236 (1989).
Newsome, W. T., Britten, K. H. & Movshon, J. A. Neuronal correlates of a perceptual decision. Nature 341, 52–54 (1989).
Abbott, L. F. Decoding neuronal firing and modeling neural networks Q. Rev. Biophys. 27, 291–331 (1994).
Abeles, M., Bergman, H., Margalit, E. & Vaadia, E. Spatiotemporal firing patterns in the frontal cortex of behaving monkeys J. Neurophysiol. 70, 1629–1638 (1993).
Gray, C. M. & Singer, W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex Proc. Natl Acad. Sci. USA 86, 1698–1702 (1989).
Roelfsma, P., Engel, A. K., König, P. & Singer, W. The role of neuronal synchronization in response selection: a biologically plausible theory of structural representations in the visual cortex J. Cog. Neurosci. 8, 603–625 (1996).
Fries, P., Roelfsma, P., Engel, A. K., König, P. & Singer, W. Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry Proc. Natl Acad. Sci. USA 94, 12699–12704 (1997).
Riehle, A., Grün, S., Diesmann, M. & Aertsen, A. Spike synchronization and rate modulation differentially involved in motor cortical function Science 278, 1950–1953 (1997).
Nicolelis, M. A. L., Baccala, L. A., Lin, R. C. S. & Chapin, J. K. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system Science 268, 1353–1358 (1995).
Milner, P. Amodel for visual shape recognition Psychol. Rev. 81, 521–535 (1974).
von der Malsburg, C. The Correlation Theory of Brain Function (Internal report, Max-Planck-Inst. Biophys. Chem., Göttingen, (1981)).
Singer, W. Synchronization of cortical activity and its putative role in information processing and learning Annu. Rev. Physiol. 55, 349–374 (1993).
Stopfer, M., Bhagavan, S., Smith, B. H. & Laurent, G. Impaired odour discrimination on desynchronization of odour-encoding assemblies. Nature 390, 70–74 (1997).
Wehr, M. & Laurent, G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature 384, 162–166 (1996).
Laurent, G. & Davidowitz, H. Encoding of olfactory information with oscillating neural assemblies Science 265, 1872–1875 (1994).
Laurent, G., Wehr, M. & Davidowitz, H. Temporal representation of odours in an olfactory network J.Neurosci. 16, 3837–3847 (1996).
MacLeod, K. & Laurent, G. Distinct mechanisms for synchronization and temporal patterning of odour-encoding neural assemblies Science 274, 976–979 (1996).
Laurent, G. & Naraghi, M. Odorant-induced oscillations in the mushroom bodies of the locust J.Neurosci. 14, 2993–3004 (1994).
Homberg, U. Processing of antennal information in extrinsic mushroom body neurons of bee brain J.Comp. Physiol. A 154, 825–836 (1984).
Li, Y. & Strausfeld, N. Morphology and sensory modality of mushroom body extrinsic neurons in the brain of the cockroach, Periplaneta americana J. Comp. Neurol. 387, 631–650 (1997).
Bacon, J. P. & Altman, J. S. Asilver intensification method for cobalt-filled neurones in wholemount preparations Brain Res. 138, 359–363 (1977).
Victor, J. D. & Purpura, K. P. Metric-space analysis of spike trains: theory, algorithms and application Network Comp. Neural. Syst. 8, 127–164 (1997).
Leitch, B. & Laurent, G. GABAergic synapses in the antennal lobe and mushroom body of the locust olfactory system J. Comp. Neurol. 373, 487–514 (1996).
Acknowledgements
We thank E. M. Schuman and C. Koch for comments on the manuscript, and the members of the Laurent laboratory for constructive criticism during this work. This work was supported by NSF and NIDCD grants (to G.L.), the Sloan Center for Neuroscience at Caltech and an NIH training grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MacLeod, K., Bäcker, A. & Laurent, G. Who reads temporal information contained across synchronized and oscillatory spike trains?. Nature 395, 693–698 (1998). https://doi.org/10.1038/27201
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/27201
This article is cited by
-
Robust encoding of natural stimuli by neuronal response sequences in monkey visual cortex
Nature Communications (2023)
-
On a Statistical Approach to Phase Synchronization in Some Map-Based Neural Chaotic Spiking–Bursting Models
Radiophysics and Quantum Electronics (2022)
-
Network mechanism for insect olfaction
Cognitive Neurodynamics (2021)
-
Molecular characterization and distribution of the voltage-gated sodium channel, Para, in the brain of the grasshopper and vinegar fly
Journal of Comparative Physiology A (2020)
-
Effects of acupuncture on neuro-electrophysiological activities in hippocampal CA1 and CA3 areas of rats with post-traumatic stress disorder
Journal of Acupuncture and Tuina Science (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.