Abstract
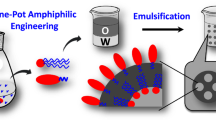
IMMOBILISATION of enzymes on solid supports to give reusable and stabilised catalysts has been studied extensively1 but the concept of chemically modifying a water-soluble enzyme to enable its activity to be utilised in the form of a water-immiscible liquid phase has received little attention. Potential advantages of liquid-phase immobilisation include ease of continuous operation, freedom from the effects of solid particle breakdown and the possibility of enhanced activity against water-insoluble liquid substrates. Although the effects of detergent-protein association on protein ex-tractability into iso-octane have been studied2 and considerable effort has been expended on the liquid membrane entrapment concept3,4, another possible type of liquid-phase immobilised enzyme system seems to have been neglected. The latter comprises droplets of a water-immiscible liquid bearing a surface layer of modified enzyme. We have found that some enzymes may be converted into surface-active amphipathic conjugates by covalent coupling to certain types of polymeric detergents (polysoaps) and that the resulting conjugates may bind sufficiently strongly to dispersed water-immiscible liquids to give an enzymatically active emulsion that can be handled as a quasi-liquid phase. The amphipathic enzyme conjugates are analogous to certain membrane-bound proteins such as cytochrome b5, which have been shown to comprise a hydrophilic protein core anchored to a lipid bilayer by a hydrophobic polypeptide tail5,6.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Zaborsky, O., Immobilised Enzymes (Chemical Rubber Co., Cleveland, Ohio, 1973).
Yonath, J., Burstein, N., and Blauer, G., Biochem. biophys. Res. Commun., 54, 507–511 (1973).
May, S. W., and Li, N. N., Biochem. biophys. Res. Commun., 47, 1179–1185 (1972).
May, S. W., and Li, N. N., in Enzyme Engineering, 2 (edit by Pye, E. K., and Wingard, L. B., Jr), (Plenum, New York and London, 1974).
Spatz, L., and Strittmatter, P. Proc natc. Acad. Sci. U.S.A., 68, 1042–1046 (1971). Spatz, L., and Strittmatter, P., J. biol. Chem., 248, 793–799 (1973).
Segrest, J. P. Kahane, I., Jackson, R. L., and Marchesi, V. T., Arch. Biochem. Biophys., 155, 167–183 (1973).
Levin, Y., Pecht, M., Goldstein, L., and Katchalski, E., Biochemistry, 3, 1905–1913 (1964).
Goldstein, L., Levin, Y., and Katchalski, E., Biochemistry, 3, 1913–1919 (1964).
Zingaro, R. A., and Uziel, M., Biochim. biophys. Acta, 213, 371–379 (1970).
Erlanger, B. G., Isambert, M. F., and Michelson, A. M., Biochem. biophys. Res. Commun., 40, 70–76 (1970).
Mosbach, K., Acta chem. Scand., 24, 2084–2902 (1970).
Patel, R. P., Lopiekes, D. V., Brown, S. P., and Price, S., Biopolymers, 5, 577–582 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SMITH, R. Amphipathic enzyme—polymer conjugates. Nature 262, 519–520 (1976). https://doi.org/10.1038/262519a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/262519a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.