Abstract
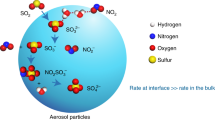
Polluted air masses are characterized by high concentrations of oxidized nitrogen compounds which are involved in photochemical smog and ozone formation. The OH radical is a key species in these oxidation processes. The photolysis of nitrous acid (HNO2), in the morning, leads to the direct formation of the OH radical and may therefore contribute significantly to the initiation of the daytime photochemistry in the polluted planetary boundary layer. But the formation of nitrous acid remains poorly understood: experimental studies imply that a suggested heterogeneous formation process involving NO2 is not efficient enough to explain the observed night-time build-up of HNO2 in polluted air masses1. Here we describe kinetic investigations which indicate that the heterogeneous production of HNO2 from NO2 on suspended soot particles proceeds 105 to 107 times faster than on previously studied surfaces. We therefore propose that the interaction between NO2 and soot particles may account for the high concentrations of HNO2 in air masses where combustion sources contribute to air pollution by soot and NOx emissions. We believe that the observed HNO2 formation results from the reduction of NO2 in the presence of water by C–O and C–H groups in the soot. Although prolonged exposure to oxidizing agents in the atmosphere is likely to affect the chemical activity of these groups, our observations nevertheless suggest that fresh soot may have a considerable effect on the chemical reactions occurring in polluted air.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lammel, G. & Cape, J. N. Nitrous acid and nitrite in the atmosphere. Chem. Soc. Rev. 361–369 (1996).
Kitto, A.-M. N. & Harrison, R. M. Nitrous and nitric acid measurements at sites in south-east England. Atmos. Environ. A 26, 235–241 (1992).
Andrés-Hernandez, M. D., Notholt, J., Hjorth, J. & Schrems, O. ADOAS study on the origin of nitrous acid at urban and non-urban sites. Atmos. Environ. 30, 175–180 (1996).
Harrison, R. M., Peak, J. D. & Collins, G. M. Tropospheric cycle of nitrous acid. J. Geophys. Res. 101, 14429–14439 (1996).
Kirchstetter, T. W., Harley, R. A. & Littlejohn, D. Measurement of nitrous acid in motor vehicle exhaust. Environ. Sci. Technol. 30, 2843–2849 (1996).
Lary, D. J.et al. Carbon aerosols and atmospheric photochemistry. J. Geophys. Res. 102, 3671–3682 (1997).
Akhter, M. S., Chughtai, A. R. & Smith, D. M. The structure of hexane soot I: Spectroscopic studies. Appl. Spectrosc. 39, 143–153 (1985).
Rogaski, C. A., Golden, D. M. & Williams, L. R. Reactive uptake and hydration experiments on amorphous carbon treated with NO2, SO2, O3, HNO3, and H2SO4. Geophys. Res. Lett. 24, 381–384 (1997).
Tabor, K., Gutzwiller, L. & Rossi, M. J. Heterogeneous chemical kinetics of NO2on amorphous carbon at ambient temperature. J. Phys. Chem. 98, 6172–6175 (1994).
Baltensperger, U.et al. Use of positron-emitting 13N for studies of the selective reduction of NO by NH3over vanadia/titania catalyst at very low reactant concentrations. J. Phys. Chem. 97, 12325–12330 (1993).
Kalberer, M.et al. Heterogeneous chemical processing of 13NO2by monodisperse carbon aerosols at very low concentrations. J. Phys. Chem. 100, 15487–15493 (1996).
Allegrini, I.et al. Annular denuder method for sampling reactive gases and aerosols in the atmosphere. Sci. Tot. Environ. 67, 1–16 (1987).
Liu, B. Y. H. & Pui, D. Y. H. Asubmicron aerosol standard and the primary absolute calibration of the condensation nuclei counter. J. Colloid Interface Sci. 47, 155–171 (1974).
Berner, A.et al. Modal character of atmospheric black carbon size distributions. J. Geophys. Res. 101, 19559–19565 (1996).
Rogak, S. N., Baltensperger, U. & Flagan, R. C. Measurement of mass transfer to agglomerate aerosols. Aerosol Sci. Technol. 14, 447–458 (1991).
Wild, U., Pfänder, N. & Schlögl, R. Species analysis of automative carbon particles: application of XPS for integral analysis of filter samles. Fresenius J. Anal. Chem. 357, 420–428 (1997).
Cachier, H., Bremond, M.-P. & Buat-Ménard, P. Determination of atmospheric soot carbon with a simple thermal method. Tellus B 41, 379–390 (1989).
Burtscher, H., Künzel, S., Hüglin, Ch. Characterization of particles in combustion engine exhaust. J.Aerosol Sci. 29, 389–396 (1998).
Chughtai, A. R., Welch, J. W. F., Akhter, M. S. & Smith, D. M. Spectroscopic study of gaseous products of soot—oxides of nitrogen/water reactions. Appl. Spectrosc. 44, 294–298 (1990).
Febo, A. & Perrino, C. Prediction and experimental evidence for high air concentration of nitrous acid in indoor environments. Atmos. Environ. A 25, 1055–1061 (1991).
Staffelbach, T., Neftel, A. & Horowitz, L. W. Photochemical oxidant formation over southern Switzerland 2. Model results. J. Geophys. Res. 102, 23363–23373 (1997).
Haugloustaine, D. A., Ridley, B. A., Solomon, S., Hess, P. G. & Madronich, S. HNO3/NOxratio in the remote troposphere during MLOPEX2: Evidence for nitric acid reduction on carbonaceous aerosols? Geophys. Res. Lett. 23, 2609–2612 (1996).
Liousse, C.et al. Aglobal three-dimensional model study of carbonaceous aerosols. J. Geophys. Res. 101, 19411–19432 (1996).
Blake, D. F. & Kato, K. Latitudinal distribution of black carbon soot in the upper troposphere and lower stratosphere. J. Geophys. Res. 100, 7195–7202 (1995).
De Santis, F. & Allegrini, I. Heterogeneous reactions of SO2and NO2on carbonaceous surfaces. Atmos. Environ. 26, 3061–3064 (1992).
Smith, D. M. & Chughtai, A. R. Photochemical effects in the heterogeneous reaction of soot with ozone at low concentrations. J. Atmos. Chem. 26, 77–91 (1997).
Weschler, C. J., Mandich, M. L. & Graedel, T. E. Speciation, photosensitivity, and reactions of transition metal ions in atmospheric droplets. J. Geophys. Res. 91, 5189–5204 (1986).
Ciccioli, P., Cecinato, A., Brancaleoni, E., Frattoni, M. & Zacchei, P. Formation and transport of 2-nitrofluoranthene and 2-nitropyrene of photochemical origin in the troposphere. J. Geophys. Res. 101, 19567–19580 (1996).
Acknowledgements
The stable proton beam from the Philips Cyclotron necessary for 13N production was provided by the staff of the Accelerator Facilities at Paul Scherrer Institute; V. Lavanchy, B. Schnyder and R. Koetz performed the offline analysis of soot samples. Contributions from S. Nyeki, C. Zellweger, E.Weingartner, P. Zimmermann and B. Eichler were greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ammann, M., Kalberer, M., Jost, D. et al. Heterogeneous production of nitrous acid on soot in polluted air masses. Nature 395, 157–160 (1998). https://doi.org/10.1038/25965
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/25965
This article is cited by
-
Synthesizing evidence for the external cycling of NOx in high- to low-NOx atmospheres
Nature Communications (2023)
-
Diesel soot photooxidation enhances the heterogeneous formation of H2SO4
Nature Communications (2022)
-
A Detailed PAH and Soot Model for Complex Fuels in CFD Applications
Flow, Turbulence and Combustion (2022)
-
Future warming exacerbated by aged-soot effect on cloud formation
Nature Geoscience (2020)
-
Global nitrous acid emissions and levels of regional oxidants enhanced by wildfires
Nature Geoscience (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.