Abstract
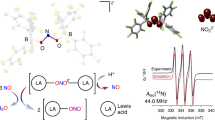
RECENT studies1 have been concerned with the mechanism of redox reactions between nitrate ions and simple mono-nuclear oxomolybdenum(V) (MovO) complexes such as [MoOCl3(OPPh3)2] to determine the function of the molybdenum centre in the nitrate reductase enzymes. In the presence of excess nitrate, the following reaction stoichiometry is obtained: MovO+NO−3→MoVIO2+NO2 (1) Kinetic studies using stopped-flow techniques identified three stages in these reactions: (1) the loss of the ligand trans to the oxo-group of the Mov complex followed by rapid, non-rate-determining coordination of nitrate at the vacated site; (2) a rearrangement of the Mov–nitrato-complex to produce a geometry suitable for rapid electron transfer resulting in the formation of NO2 and a cis-dioxomolybdenumVI (MoVIO2) complex; (3) ligand substitution(s) at the cis-MoVIO2 centre.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Garner, C. D., Hyde, M. R., Mabbs, F. E., and Routledge, V. I., Nature, 252, 579–580 (1974).
Bamford, C. H., and Tipper, C. F. N., Chemical Kinetics, 7, 23, (Elsevier, Amsterdam, 1972).
Latimer, W. M., Oxidation States of the Elements and their Potentials in Aqueous Solutions, second ed., 104 (Prentice-Hall, New York, 1952).
Forget, P., and Dervartanian, D. V., Biochim. biophys. Acta, 256, 600–606 (1972).
Frank, J. A., and Spence, J. T., J. phys. Chem., 68, 2131–2135 (1964).
Norbury, A. H., and Sinha, A. I. P., Q. Rev. 24, 69–94 (1970).
Gillette, R. H., and Eyster, E. H., Phys. Rev., 56, 1113–1119 (1939).
Vogel, A. I., Quantitative Inorganic Analysis, second ed., 285 (Longmans, London, 1953).
Pfeiffer, G. V., and Allen, L. C., J. chem. Phys., 51, 190–202 (1969).
Gray, H. B., and Hare, C. R., Inorg. Chem., 1, 363–368 (1962).
Taube, H., J. chem. Educ., 45, 452–461 (1968).
Murmann, R. K., and Taube, H., J. Am. chem. Soc., 78, 4886 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GARNER, C., HYDE, M. & MABBS, F. Possible mechanism for discrimination between nitrate and nitrite by nitrate reductases. Nature 253, 623–625 (1975). https://doi.org/10.1038/253623a0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/253623a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.