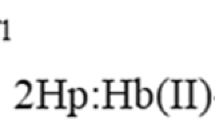Abstract
AMONG the large number of human haemoglobin variants of known structure, most are functionally normal. Haemoglobin Chesapeake (α2 92 Arg→Leu β2) is the first one found in association with elevated whole blood oxygen affinity and familial erythrocytosis. By contrast with haemoglobin A, isolated haemoglobin Chesapeake displays a six-fold increase in oxygen affinity and a marked reduction in haem–haem interaction (Hill's coefficient n=1.3), but a normal Bohr effect2. In the past two years, other variants with similar properties have been reported, but none has been studied as extensively as haemoglobin Chesapeake. These mutants provide considerable information relating the structure and function of haemoglobin, for their amino-acid substitutions are likely to be at sites crucial to the normal function of the molecule. It is of considerable interest that in four of these haemoglobins (Chesapeake, J-Capetown, Yakima and Kempsey), the substitutions are located in the area of contact between the α1 and β2 chains3. Three-dimensional models constructed from the X-ray crystallographic data of Perutz and associates indicate that, during oxygenation, there is considerable movement of the β chains relative to the α chains at this interface4. It is likely that the symmetrical dissociation of oxyhaemoglobin into αβ dimers also occurs at the α1β2 contact areas5,6.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Charache, S., Weatherall, D. J., and Clegg, J. B., J. Clin. Invest., 45, 813 (1966).
Nagel, R. L., Gibson, Q. H., and Charache, S., Biochemistry, 6, 2395 (1967).
Perutz, M. F., and Lehmann, H., Nature, 219, 902 (1968).
Muirhead, H., Cox, J. M., Mazzarella, L., and Perutz, M. F., J. Mol. Biol., 28, 117 (1967).
Perutz, M. F., Muirhead, H., Cox, J. M., and Goaman, L. C. G., Nature, 219, 131 (1968).
Rosemeyer, M. A., and Huehns, E. R., J. Mol. Biol., 25, 253 (1967).
Bunn, H. F., J. Clin. Invest., 48, 126 (1969).
Dalziel, K., and O'Brien, J. R., Biochem. J., 67, 119 (1957).
Benesch, R. E., Benesch, R., and Williamson, M. E., Proc. US Nat. Acad. Sci., 48, 2071 (1962).
Guidotti, G., J. Biol. Chem., 242, 3685 (1967).
Edelstein, S., and Gibson, Q. H., in Heme and Hemoproteins, Proc. Fifth Colloq. Johnson Research Foundation Univ. Pennsylvania (in the press, 1969).
Yphantis, D. A., Biochemistry, 3, 297 (1964).
Bucci, E., and Fronticelli, C., J. Biol. Chem., 240, PC 551 (1965).
Wyman, J., Adv. Protein Chem., 19, 223 (1964).
Guidotti, G., J. Biol. Chem., 242, 3704 (1967).
Noble, R. W., J. Mol. Biol., 39, 479 (1969).
Greer, J., Bunn, H. F., Ho, C., and Charache, S., Blood, 34, 838 (1969).
Ho, C., Davis, D. G., Mock, N. H., Lindstrom, T. R., and Charache, S., Biochem. Biophys. Res. Commun., 38, 779 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BUNN, H. Dissociation of Haemoglobin Chesapeake into Subunits. Nature 227, 839–840 (1970). https://doi.org/10.1038/227839a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/227839a0
This article is cited by
-
Extensions of the Allosteric Model for Haemoglobin
Nature (1971)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.