Abstract
The adverse effects of extra-erythrocytic hemoglobin (Hb) are counterbalanced by several plasma proteins devoted to facilitate the clearance of free heme and Hb. In particular, haptoglobin (Hp) traps the αβ dimers of Hb, which are delivered to the reticulo-endothelial system by CD163 receptor-mediated endocytosis. Since Hp:Hb complexes show heme-based reactivity, kinetics of O2 dissociation from the ferrous oxygenated human Hp1-1:Hb and Hp2-2:Hb complexes (Hp1-1:Hb(II)-O2 and Hp2-2:Hb(II)-O2, respectively) have been determined. O2 dissociation from Hp1-1:Hb(II)-O2 and Hp2-2:Hb(III)-O2 follows a biphasic process. The relative amplitude of the fast and slow phases ranges between 0.47 and 0.53 of the total amplitude, with values of koff1 (ranging between 25.6 ± 1.4 s−1 and 29.1 ± 1.3 s−1) being about twice faster than those of koff2 (ranging between 13.8 ± 1.6 s−1 and 16.1 ± 1.2 s−1). Values of koff1 and koff2 are essentially the same independently on whether O2 dissociation has been followed after addition of a dithionite solution or after O2 displacement by a CO solution in the presence of dithionite. They correspond to those reported for the dissociation of the first O2 molecule from tetrameric Hb(II)-O2, indicating that in the R-state α and β chains are functionally heterogeneous and the tetramer and the dimer behave identically. Accordingly, the structural conformation of the α and β chains of the Hb dimer bound to Hp corresponds to that of the subunits of the Hb tetramer in the R-state.
Similar content being viewed by others
Introduction
Hemoglobin (Hb), the most prominent intracellular circulating protein, is devoted to the O2 transport and the chemistry of reactive oxygen and nitrogen species1,2,3,4,5,6,7. Physiologically, Hb is released in plasma during the enucleation of erythroblasts and the hemolysis of senescent erythrocytes. Moreover, the intravascular release of Hb occurs during blood transfusion and represents a dramatic pathological complication of several diseases, including autoimmune, infectious, and inherited diseases1,8,9,10. The toxicity of extra-erythrocytic Hb depends on: (i) the heme-Fe-based production of free radicals, which induces the lipids peroxidation triggering the inflammatory cascade11, (ii) the sequestering of NO produced by endothelial cells lowering its bioavailability12, and (iii) the effects in the kidneys due to its renal filtration13.
The adverse effects of extra-erythrocytic Hb are counterbalanced by several plasma proteins devoted to facilitate the clearance of free heme and Hb. In particular, high and low density lipoproteins, albumin, and hemopexin ensure the complete clearance of the free heme, which is released into hepatic parenchymal cells by the CD91 receptor-mediated endocytosis of the hemopexin-heme complex. After conveying the heme intracellularly, hemopexin is released into the bloodstream and the heme is degraded9,14,15,16. Moreover, haptoglobin (Hp) is devoted to trap the extra-erythrocytic αβ dimers of Hb; the formation of Hb dimers, and in turn of the Hp:Hb complexes, is favored by the low extra-erythrocytic Hb concentration and its oxygenated state. By CD163 receptor-mediated endocytosis, the Hp:Hb complexes are delivered to the reticulo-endothelial system where they are degraded to release the heme. Heme-oxygenase catalyzes the heme conversion to biliverdin that is further transformed to bilirubin. The bilirubin is then exported from the macrophage and carried from albumin to the liver for conjugation in the hepatocytes and subsequent biliary excretion9,15,16,17,18,19,20,21,22.
In humans, two alleles of Hp (Hp1 and Hp2) are expressed as single polypeptide chains. In particular, Hp1 displays a single complement control protein (CCP) domain and a single serine protease-like (SP-like) domain, whereas Hp2 contains two CCP domains and one SP-like domain23. Both Hp1 and Hp2 are proteolytically cleaved into α and β chains, which are covalently linked by a disulfide bond(s). Hp1 and Hp2 alleles induce the formation of Hp1-1 dimers (covalently linked by Cys15 residues), Hp1-2 hetero-oligomers and Hp2-2 oligomers (covalently linked by Cys15 and Cys74 residues)21. The most abundant Hp2-2 species is the tetramer, but trimers and higher order oligomers have been reported22,23,24,25.
Each Hp β chain binds one αβ dimer of Hb, making extensive contacts with the Hb dimer-dimer interface24,25. Accordingly, values of (i) the dissociation equilibrium constants for the recognition of deoxygenated and oxygenated Hb (Hb(II) and Hb(II)-O2, respectively) dimers by Hp are 1 × l0−7 M and 1.3 × l0−6 M, respectively, and (ii) the second order rate constants for Hp:Hb complexation range between 5 × 105 M−1 s−1 and 9 × 105 M−1 s−1 26,27,28. Since Hb(II)-O2 dissociates into αβ dimers preferentially with respect to Hb(II), the reaction of Hp with Hb represents a probe of the R-T transition of Hb27,29.
As the Hp:Hb complexes show functional properties similar to those of the Hb R-state, e.g. they display a high ligand specificity and show neither “heme-heme interactions” nor the Bohr effect30,31,32,33,34,35,36,37, we decided to investigate the kinetics of O2 dissociation from human Hb(II)-O2 dimers bound to human Hp phenotypes 1-1 and 2-2 (Hp1-1:Hb(II)-O2 and Hp2-2:Hb(II)-O2, respectively). The relevance of this approach is related to the fact that in this way it is possible to characterize the O2 dissociation from a pure population of α1β1 (and α2β2) dimers without any interference from tetrameric species. Therefore, it is possible to sort out the contribution of the α1β1 (and α2β2) inter-subunit contacts from the α1β2 (and α2β1) ones, which are destroyed upon dimerization38. O2 dissociation from Hp1-1:Hb(II)-O2 and Hp2-2:Hb(II)-O2 follows a biphasic process, the fast process (i.e., koff1 values) being about 2-fold faster than the slow one (i.e., koff2 values). Values of koff1 and koff2 are similar to those for the deoxygenation of isolated α(II)-O2 and β(II)-O2 chains of Hb39,40 and identical to those for the dissociation of the first O2 molecule from tetrameric Hb(II)-O241. The close similarity for the observed heterogeneity between experiments of O2 replacement by CO and those of O2 dissociation by sodium dithionite allows to state unequivocally for the first time that the biphasicity cannot be referable to a negative cooperativity in the α1β1 (and α2β2) dimers. It clearly demonstrates that the α and β chains of the oxygenated R-state of Hb are functionally heterogeneous to the same extent both in the tetrameric and in the dimeric assembly. Accordingly, the conformation of the α and β chains of the Hb dimer bound to Hp corresponds to that of the α1β1 (and α2β2) dimers in the R-state tetramer24,25,42,43.
Materials
Human Hp1-1 and Hp2-2 were purchased from Athens Research & Technology, Inc. (Athens, GA, USA). Human oxygenated Hb was prepared as previously reported44. The oxygenated Hp:Hb complexes were prepared by mixing oxygenated Hb with Hp1-1 and Hp2-2 at pH 7.0 and 20.0 °C29. The dimeric Hp:tetrameric Hb stoichiometry was 1:1. To avoid the occurrence of free Hb, a 20% excess of Hp1-1 and Hp2-2 was present in all samples. The absence of free Hb was checked by gel electrophoresis31.
CO was purchased from Linde AG (Höllriegelskreuth, Germany). The CO solution was prepared by keeping in a closed vessel the 5.0 × 10−2 M phosphate buffer solution (pH = 7.0) under CO at P = 760.0 mm Hg anaerobically (T = 20.0 °C). The solubility of CO in the aqueous buffered solution is 1.03 × 10−3 M, at P = 760.0 mm Hg and T = 20.0 °C44.
All the other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals were of analytical grade and were used without further purification.
Methods
Kinetics of O2 dissociation from Hp1-1:Hb(II)-O2 and Hp2-2:Hb(II)-O2 (final concentration, 3.2 × 10−6 M to 5.5 × 10−6 M) were investigated either by mixing oxygenated Hp:Hb(II) complexes with a dithionite solution (final concentration, 1.0 × 10−3 M)33 or by O2 replacement with CO in the presence of dithionite (final concentrations, 5.0 × 10−4 M and 3.0 × 10−3 M, respectively)41. No gaseous phase was present.
Since with both methods, the O2 dissociation time courses from Hp1-1:Hb(II)-O2 and Hp2-2:Hb(II)-O2 display two exponentials, they have been analyzed in the framework of either Fig. 1 or Fig. 2.
Values of the apparent first-order rate constants for O2 dissociation from Hp1-1:Hb(II)-O2 and Hp2-2:Hb(II)-O2 (i.e., koff1 and koff2) were obtained according to Eqs 1 and 245:
depending on the observation wavelength. Hp:Hb(II)-O2 indicates either Hp1-1:Hb(III)-O2 or Hp2-2:Hb(III)-O2, and a and b indicate the relative amplitude of the fast and slow binding process, respectively (i.e., a + b = 1; i.e., 100%).
O2 dissociation from Hp1-1:Hb(II)-O2 and Hp2-2:Hb(II)-O2 was investigated at pH 7.0 (5.0 × 10−2 phosphate buffer) and 20.0 °C. Kinetics was monitored by single-wavelength stopped-flow spectroscopy between 380 and 460 nm. The amplitude of the time courses for conversion of Hp:Hb(II)-O2 to Hp:Hb(II)-CO and of Hp:Hb(II)-O2 to Hp:Hb(II) was normalized to 420 and 430 nm, respectively. All kinetic experiments have been carried out with the BioLogic SFM-200 rapid-mixing stopped-flow apparatus (Claix, France); the dead-time of the stopped-flow apparatus was 1.4 ms and the observation chamber was 1 cm.
The results (from at least four experiments) are given as mean values plus or minus the corresponding standard deviation. All data were analyzed using the GraphPad Prism program, version 5.03 (GraphPad Software, La Jolla, CA, USA).
Comparison of the three-dimensional structure of the αβ dimer of Hb bound to Hp (PDB code 5JDO; resolution 3.2 Å)43 with the corresponding subunits of the Hb tetramer in the R-state (PDB code 2DN1; resolution 1.25 Å)42, including calculation of root mean square deviation (rmsd) values, has been carried out using SwissPDBViewer46.
Results and Discussion
Under all the experimental conditions, O2 dissociation from Hp1-1:Hb(II)-O2 and Hp2-2:Hb(III)-O2 follows a biphasic process upon mixing the Hp1-1:Hb(II)-O2 and Hp2-2:Hb(III)-O2 solutions either with the dithionite solution or the CO solution in the presence of dithionite (Fig. 3). This is supported by statistical analysis (Table 1) and the residual distribution shown in Fig. 3 of the Supplementary Information section.
Average time course of O2 dissociation from Hp1-1:Hb(II)-O2 (panels A and C) and from Hp2-2:Hb(II)-O2 (panels B and D) by mixing the Hp-:Hb(II)-O2 solutions with the CO solution in the presence of dithionite (panels A and B, respectively) and with the dithionite solution only (panels C and D). The continuous lines were calculated according to Eq. 2 with the following parameters: (panel A) a = 0.49, koff1 = 27.4 s−1, b = 0.51, and koff2 = 15.1 s−1; (panel B) a = 0.47, koff1 = 26.3 s−1, b = 0.53, and koff2 = 13.1 s−1; (panel C) a = 0.52, koff1 = 28.3 s−1, b = 0.48, and koff2 = 14.2 s−1; and (panel D) a = 0.49, koff1 = 28.8 s−1, b = 0.51, and koff2 = 12.6 s−1.
The relative amplitude of the fast and slow phases (i.e., values of a and b, respectively; see Eqs 1 and 2) ranges between 0.47 and 0.53 of the total amplitude (i.e., a + b = 1) over the whole wavelength range explored (i.e., between 380 and 460 nm). Moreover, the difference absorbance spectra of the fast and the slow phases of Hp1-1:Hb(II)-O2 minus Hp1-1:Hb(II)-CO overlap with those of Hp2-2:Hb(II)-O2 minus Hp2-2:Hb(II)-CO and the difference absorbance spectra of the fast and the slow phases of Hp1-1:Hb(II)-O2 minus Hp1-1:Hb(II) are superimposable to those of Hp2-2:Hb(II) minus Hp2-2:Hb(II)-CO. In turn, they display the same shape of the overall difference absorbance spectra of Hp1-1:Hb(II)-O2 minus Hp1-1:Hb(II)-CO and Hp2-2:Hb(II)-O2 minus Hp2-2:Hb(II)-CO, and of Hp1-1:Hb(II)-O2 minus Hp1-1:Hb(II) and Hp2-2:Hb(II)-O2 minus Hp2-2:Hb(II) (Fig. 4). The fast process of Hp1-1:Hb(II)-O2 and Hp2-2:Hb(II)-O2 deoxygenation is about 2-fold faster than the slow one with values of koff1 ranging between 25.6 ± 1.4 s−1 and 29.1 ± 1.3 s−1 and koff2 ranging between 13.8 ± 1.6 s−1 and 16.1 ± 1.2 s−1. Moreover, values of koff1 and koff2 are closely similar for the Hp1-1:Hb(II)-O2 and Hp2-2:Hb(II)-O2 species (Table 1).
Difference absorbance spectra of the fast (circles) and the slow (squares) phases of Hp1-1:Hb(II)-O2 minus Hp1-1:Hb(II)-CO (panel A), Hp1-1:Hb(II)-O2 minus Hp1-1:Hb(II) (panel B), Hp2-2:Hb(II)-O2 minus Hp2-2:Hb(II)-CO (Panel C), and Hp2-2:Hb(II)-O2 minus Hp2-2:Hb(II) (panel D). Triangles indicate the total difference absorbance spectra. The relative amplitude of the fast and slow phases ranges between 0.47 and 0.53 over the whole wavelength range explored (i.e., between 380 and 460 nm).
Interestingly, values of koff1 and koff2 are closely similar to both those for the deoxygenation of isolated α(II)-O2 and β(II)-O2 chains of Hb (28 ± 6 s−1 and 16 ± 5 s−1, respectively39; and 28 ± 3 s−1 and 18 ± 2 s−1, respectively40) and those for the dissociation of the first O2 molecule from tetrameric Hb(II)-O2 (21.2 ± 2.6 s−1 and 13.0 ± 1.4 s−1, respectively)41 (Table 1). Of note, while for isolated chains the O2 dissociation rate constant is faster for the α-chains39, in the case of tetrameric Hb(II)-O2 the dissociation of the first O2 molecule from the tetra-ligated species seems to be faster for the β-subunit41. This assembly-linked subunit functional difference has been suggested to reflect a structural change(s) of the α-chain upon the tetrameric assembly leading to a slower O2 dissociation rate constant. This finding has been also confirmed by subsequent observations on the kinetics of O2 displacement from oxygenated Hb by CO47,48, where the subunit characterized by the faster phase displays a higher affinity for organic phosphate, which is known to bind at the β-dyad axis49,50. Therefore, it seems very reasonable to identify the β-subunit as the one characterized by the faster O2 dissociation rate in the tetrameric R-state of Hb.
In the case of the Hp:Hb(II)-O2 complexes, a different situation occurs since Hp binds the α1β1 (and the α2β2) dimers24,25; therefore, parameters here reported (Table 1) reflect the functional features of this dimeric structure. In a previous paper on the O2 binding properties of the Hp2-2:Hb(II) complex33 the biphasic O2 dissociation process was detected, but a two-fold higher rate was reported for the fast phase (i.e., 59.5 ± 5.5 s−1) whereas the slower rate is closely similar to what observed by us (Table 1). At the moment, we have no obvious explanation for this discrepancy between data here described and previous ones33, but indeed the close similarity for O2 dissociation rates by both (i) mixing Hp:Hb(II)-O2 complexes with dithionite (thus fully deoxygenating Hp:Hb(II)-O2 to Hp:Hb(II), see Fig. 1) and (ii) displacing O2 with CO (thus keeping always a fully liganded form, see Fig. 2), as observed by us (Table 1), rules out the possibility of a negative cooperativity within the α1β1 dimer, definitely assigning the biphasicity to a different kinetic behavior of the two subunits.
Further, data shown in Table 1 confirm the view that: (i) the αβ dimers of Hb(II)-O2 bound to Hp1-1 and Hp2-2 are in the R-state as reported for isolated α(II)-O2 and β(II)-O2 chains and (ii) Hp1-1 and Hp2-2 species affect to the same extent the O2 dissociation from Hp1-1:Hb(II)-O2 and Hp2-2:Hb(III)-O2. In fact, the two CCP domains present in each Hp monomer are involved in the protein dimerization and do not participate to the recognition of the αβ dimers of Hb(II)-O223,24,25.
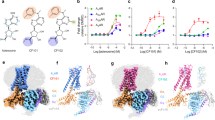
Lastly, the similar reactivity of the αβ dimers of Hb(II)-O2 bound to Hp and of the tetrameric R-state is in keeping with structural data. Indeed, both subunits of the αβ dimers of Hb(II)-O2 bound to Hp43 display a conformation superimposable to that of the α and β chains of tetrameric oxygenated Hb with the Fe(II) atom positioned in the plane of the heme group42 (Fig. 5). This is confirmed by the very low backbone rmsd value (0.56 Å) calculated upon best fitting the three-dimensional structure of the αβ dimer of Hb bound to Hp (PDB code 5JDO)43 to the corresponding subunits of the Hb tetramer in the R-state (PDB code 2DN1)42.
Structural superposition of the three-dimensional structure of the αβ dimer of Hb bound to Hp (cyan; PDB code 5JDO)43 to the corresponding subunits of the Hb tetramer in the R-state (light brown; PDB code 2DN1)42. The heme-Fe moieties are shown in stick representation. Iron atoms are represented by orange spheres and O2 molecules as red sticks. The figure has been drawn using the UCSF Chimera software52.
A final consideration is required with respect to the consequences of these data for the comprehension of the cooperative mechanism of O2 binding to Hb. It has been shown that the ligand-linked quaternary structural change, responsible for the cooperativity, is connected to a shift of the α1β2 (as well as of α2β1) interfaces2,51, as also suggested by the fact that at very high ionic strength the dissociation into α1β1 (and α2β2) dimers brings about the disappearance of cooperativity38, indicating that dimers are in a R-like quaternary state. However, the evidence of a functional heterogeneity for the O2 dissociation in the R state41,48 raised the question on the role of the α1β1 (and α2β2) subunit interaction in the energetics of cooperativity. Although the stabilization of the dimeric assembly upon interaction of Hb with Hp has been established since long time27 and a kinetic heterogeneity for the O2 dissociation has been shown also for the Hp:Hb complex33, it is only the close similarity, reported in this paper for the first time, between the O2 dissociation rates measured by full deoxygenation and O2 displacement by CO (Fig. 3) that allows to state that no ligand-linked structural change occurs at the α1β1 (and α2β2) subunit interaction surface, as also supported by the substantial identity for the dimer structure in the Hp:Hb complex and in the tetrameric oxygenated Hb (Fig. 5).
References
Bunn, H. F. & Forget, B. G. Hemoglobin: Molecular, Genetic and Clinical Aspects. Saunders, Philadelphia, PA, 1986.
Perutz, M. F. Mechanisms regulating the reactions of human hemoglobin with oxygen and carbon monoxide. Annu. Rev. Physiol. 52, 1–25 (1990).
Brunori, M. Hemoglobin is an honorary enzyme. Trends Biochem. Sci. 24, 158–161 (1999).
Gow, A. J., Luchsinger, B. P., Pawloski, J. R., Singel, D. J. & Stamler, J. S. The oxyhemoglobin reaction of nitric oxide. Proc. Natl. Acad. Sci. USA 96, 9027–9032 (1999).
Imai, K. The haemoglobin enzyme. Nature 401, 437–439 (1999).
McMahon, T. J. et al. Nitric oxide in the human respiratory cycle. Nat. Med. 8, 711–717 (2002).
Ascenzi, P. & Brunori, M. A molecule for all seasons: the heme. J. Porphyrins Phthalocyanines 20, 134–149 (2016).
Muller-Eberhard, U., Javid, J., Liem, H. H., Hanstein, A. & Hanna, M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood 32, 811–815 (1968).
Ascenzi, P. et al. Hemoglobin and heme scavenging. IUBMB Life 57, 749–759 (2005).
Alayash, A. I., Andersen, C. B., Moestrup, S. K. & Bülow, L. Haptoglobin: the hemoglobin detoxifier in plasma. Trends Biotechnol. 31, 2–3 (2013).
Dutra, F. F. & Bozza, M. T. Heme on innate immunity and inflammation. Front. Pharmacol. 5, 115 (2014).
Alayash, A. I. Oxygen therapeutics: can we tame haemoglobin? Nat. Rev. Drug Discov. 3, 152–159 (2004).
Fagoonee, S. et al. Plasma protein haptoglobin modulates renal iron loading. Am. J. Pathol. 166, 973–983 (2005).
Miller, Y. I. & Shaklai, N. Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim. Biophys. Acta 1454, 153–164 (1999).
Nielsen, M. J., Møller, H. J. & Moestrup, S. K. Hemoglobin and heme scavenger receptors. Antioxid. Redox Signal. 12, 261–273 (2010).
Smith, A. & McCulloh, R. J. Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders. Front. Physiol. 6, 187 (2015).
Kristiansen, M. et al. Identification of the haemoglobin scavenger receptor. Nature 409, 198–201 (2001).
Buehler, P. W. et al. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood 113, 2578–2586 (2009).
Levy, A. P. et al. Haptoglobin: basic and clinical aspects. Antioxid. Redox Signal. 12, 293–304 (2010).
Ratanasopa, K., Chakane, S., Ilyas, M., Nantasenamat, C. & Bulow, L. Trapping of human hemoglobin by haptoglobin: molecular mechanisms and clinical applications. Antioxid. Redox Signal. 18, 2364–2374 (2013).
Schaer, D. J., Vinchi, F., Ingoglia, G., Tolosano, E. & Buehler, P. W. Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development. Front. Physiol. 5, 415 (2014).
Andersen, C. B. F. et al. Haptoglobin. Antioxid. Redox Signal. 26, 814–831 (2017).
Polticelli, F., Bocedi, A., Minervini, G. & Ascenzi, P. Human haptoglobin structure and function - a molecular modelling study. FEBS J. 275, 5648–5656 (2008).
Andersen, C. B. et al. Structure of the haptoglobin-haemoglobin complex. Nature 489, 456–459 (2012).
Stødkilde, K., Torvund-Jensen, M., Moestrup, S. K. & Andersen, C. B. Structural basis for trypanosomal haem acquisition and susceptibility to the host innate immune system. Nat. Commun. 5, 5487 (2014).
Andersen, M. E., Moffat, J. K. & Gibson, Q. H. The kinetics of ligand binding and of the association-dissociation reactions of human hemoglobin. Properties of deoxyhemoglobin dimers. J. Biol. Chem. 246, 2796–2807 (1971).
Nagel, R. L. & Gibson, Q. H. The binding of hemoglobin to haptoglobin and its relation to subunit dissociation of hemoglobin. J. Biol. Chem. 246, 69–73 (1971).
Mollan, T. L. et al. Redox properties of human hemoglobin in complex with fractionated dimeric and polymeric human haptoglobin. Free Radic. Biol. Med. 69, 265–277 (2014).
Nagel, R. L. & Gibson, Q. H. The hemoglobin-haptoglobin reaction as a probe of hemoglobin conformation. Biochem. Biophys. Res. Commun. 48, 959–966 (1972).
Nagel, R. L. & Gibson, Q. H. Kinetics of the reaction of carbon monoxide with the hemoglobin-haptoglobin complex. J. Mol. Biol. 22, 249–255 (1966).
Brunori, M., Alfsen, A., Saggese, U., Antonini, E. & Wyman, J. Studies on the oxidation-reduction potentials of heme proteins. VII. Oxidation-reduction equilibrium of hemoglobin bound to haptoglobin. J. Biol. Chem. 243, 2950–2954 (1968).
Sawicki, C. A. & Gibson, Q. H. Quaternary conformational changes in human hemoglobin studied by laser photolysis of carboxyhemoglobin. J. Biol. Chem. 251, 1533–1542 (1976).
Chiancone, E., Antonini, E., Brunori, M., Alfsen, A. & Lavialle, F. Kinetics of the reaction between oxygen and haemoglobin bound to haptoglobin. Biochem. J. 133, 205–207 (1973).
Azarov, I. et al. Rate of nitric oxide scavenging by hemoglobin bound to haptoglobin. Nitric Oxide 18, 296–302 (2008).
Ascenzi, P. & Coletta, M. Peroxynitrite detoxification by human haptoglobin:hemoglobin complexes: a comparative study. J. Phys. Chem. B, https://doi.org/10.1021/acs.jpcb.8b05340 (2018).
Ascenzi, P., De Simone, G., Polticelli, F., Gioia, M. & Coletta, M. Reductive nitrosylation of ferric human hemoglobin bound to human haptoglobin 1-1 and 2-2. J. Biol. Inorg. Chem. 23, 437–445 (2018).
Ascenzi, P., Tundo, G. R. & Coletta, M. The nitrite reductase activity of ferrous human hemoglobin:haptoglobin 1-1 and 2-2 complexes. J. Inorg. Biochem. 187, 116–122 (2018).
Hewitt, J. A., Kilmartin, J. V., Eyck, L. F. & Perutz, M. F. Non-cooperativity of the αβ dimer in the reaction of hemoglobin with oxygen. Proc. Natl. Acad. Sci. USA 69, 203–207 (1972).
Brunori, M., Noble, R. W., Antonini, E. & Wyman, J. The reaction of isolated α and β chains of human hemoglobin with oxygen and carbon monoxide. J. Biol. Chem. 241, 5238–5243 (1966).
Olson, J. S., Rohlfs, R. J. & Gibson, Q. H. Ligand recombination to the α and β subunits of human hemoglobin. J. Biol. Chem. 262, 12930–12938 (1987).
Olson, J. S., Andersen, M. E. & Gibson, Q. H. The dissociation of the first oxygen molecule from some mammalian oxyhemoglobins. J. Biol. Chem. 246, 5919–5923 (1971).
Park, S.-Y., Yokoyama, T., Shibayama, N., Shiro, Y. & Tame, J. R. H. 1.25 Å resolution crystal structures of human haemoglobin in the oxy, deoxy and carbonmonoxy forms. J. Mol. Biol. 360, 690–701 (2006).
Lane-Serff, H. et al. Evolutionary diversification of the trypanosome haptoglobin-haemoglobin receptor from an ancestral haemoglobin receptor. Elife 5, e13044 (2016).
Antonini, E. & Brunori, M., Hemoglobin and Myoglobin in their Reactions of with Ligands. North Holland Publishing Co., Amsterdam, 1971.
Gibson, Q. H., Parkhurst, L. J. & Geraci, G. The reaction of methemoglobin with some ligands. J. Biol. Chem. 244, 4668–4676 (1969).
Guex, N. & Peitsch, M. C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Coletta, M., Ascenzi, P., Santucci, R., Bertollini, A. & Amiconi, G. Interaction of inositol hexakisphosphate with liganded ferrous human hemoglobin. Direct evidence for two functionally operative binding sites. Biochim. Biophjys. Acta 1162, 309–314 (1993).
Coletta, M., Ascenzi, P., Castagnola, M. & Giardina, B. Functional and spectroscopic evidence for a conformational transition in ferrous liganded human hemoglobin. J. Mol. Biol. 249, 800–803 (1995).
Arnone, A. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature 237, 146–149 (1972).
Arnone, A. & Perutz, M. F. Structure of inositol hexaphosphate-human deoxyhaemoglobin complex. Nature 249, 34–36 (1974).
Ackers, G. K., Doyle, M. L., Myers, D. & Daugherty, M. A. Molecular code for cooperativity in hemoglobin. Science 255, 54–63 (1992).
Pettersen, E. F. et al. UCSF Chimera - a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
This paper is dedicated to Prof. Emilia Chiancone, deceased on December 18th 2018, who pioneered structure-function relationships of high molecular weight heme-proteins. Molecular graphics and analyses were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311. The grant of Dipartimenti di Eccellenza, MIUR (Legge 232/2016, Articolo 1, Comma 314–337) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
P.A., made substantial contributions to study concept and design, performed the experiments, contributed to data analysis and manuscript drafting; F.P., contributed to data analysis and manuscript drafting; M.C., made substantial contributions to study concept and design, contributed to data analysis and manuscript drafting.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ascenzi, P., Polticelli, F. & Coletta, M. Oxygen dissociation from ferrous oxygenated human hemoglobin:haptoglobin complexes confirms that in the R-state α and β chains are functionally heterogeneous. Sci Rep 9, 6780 (2019). https://doi.org/10.1038/s41598-019-43190-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43190-x
This article is cited by
-
Kinetics of cyanide and carbon monoxide dissociation from ferrous human haptoglobin:hemoglobin(II) complexes
JBIC Journal of Biological Inorganic Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.