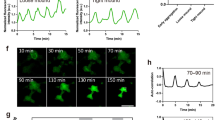
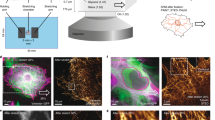
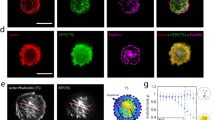
Abstract
Intracellular calcium regulates many of the molecular processes that are essential for cell movement1. It is required for the production of actomyosin-based contractile forces2,3,4, the regulation of the structure and dynamics of the actin cytoskeleton5,6, and the formation and disassembly of cell–substratum adhesions7,8. Calcium also serves as a second messenger in many biochemical signal-transduction pathways7. However, despite the pivotal role of calcium in motile processes, it is not clear how calcium regulates overall cell movement. Here we show that transient increases in intracellular calcium, [Ca2+]i, during the locomotion of fish epithelial keratocytes, occur more frequently in cells that become temporarily ‘stuck’ to the substratum or when subjected to mechanical stretching. We find that calcium transients arise from the activation of stretch-activated calcium channels, which triggers an influx of extracellular calcium. In addition, the subsequent increase in [Ca2+]i is involved in detachment of the rear cell margin. Thus, we have defined a mechanism by which cells can detect and transduce mechanical forces into biochemical signals that can modulate locomotion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stossel, TP. On the crawling of animal cells. Science 260, 1086–1094 (1993).
Strohmeier, R. & Bereiter-Hahn, J. Control of cell shape and locomotion by external calcium. Exp. Cell Res. 154, 412–420 (1984).
Citi, S. & Kendrick-Jones, J. Regulations of non-muscle myosin structure and function. BioEssays 7, 155–159 (1987).
Rees, D. A.et al. Myosin regulation and calcium transients in fibroblast shape change: Attachment and patching. Cell Motil. Cytoskel. 13, 112–122 (1989).
Condeelis, J. Life at the leading edge: The formation of cell protrusions. Annu. Rev. Cell Biol. 9, 411–444 (1993).
Hartwig, J. H. & Yin, H. The organization and regulation of the macrophage actin skeleton. Cell Motil. Cytoskel. 10, 117–125 (1988).
Sjaastad, M. D. & Nelson, W. J. Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. BioEssays 19, 47–55 (1997).
Crowley, E. & Horwitz, A. F. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J. Cell Biol. 131, 525–537 (1995).
Lee, J. & Jacobson, K. The composition and dynamics of cell-substratum adhesions in locomoting fish keratocytes. J. Cell Sci. 110, 2833–2844 (1997).
Svitkina, T. M., Verkhovsky, A. B., McQuade, K. M. & Borisy, G. G. Analysis of the actin-myosin II system in fish epidermal keratocytes: Mechanism of cell body translocation. J. Cell Biol. 139, 397–415 (1997).
Galbraith, CG. & Sheetz, M. P. Relationship between cell shape, traction force, and speed. Mol. Biol. Cell(suppl.) 8, 385 (1997).
Anderson, K. I., Wang, Y.-L. & Small, J. V. Coordination of protrusion and translocation of the keratocyte involves rolling of the cell body. J. Cell Biol. 134, 1209–1218 (1996).
Lee, J., Leonard, M., Oliver, T., Ishihara, A. & Jacobson, K. Traction forces generated by locomoting keratocytes. J. Cell Biol. 127, 1957–1964 (1994).
Lee, J., Ishihara, A., Theriot, J. A. & Jacobson, K. Principles of locomotion for simple-shaped cells. Nature 362, 167–171 (1993).
Theriot, J. A. & Mitchison, T. J. Actin microfilament dynamics in locomoting cells. Nature 352, 126–131 (1991).
Sachs, F. in Sensory Transduction (eds Corey, D P. & Roper, S.) 242–260 (Rockefeller University Press, New York, 1992).
Gustin, M. C., Zhou, X., Martinac, B. & Kung, C. Amechanosensitive ion channel in the yeast plasma membrane. Science 242, 762–765 (1988).
Hamill, O. P. & McBride, D. W. Rapid adaptation of single mechanosensitive channels in Xenopus oocytes. Proc. Natl Acad. Sci. USA 89, 7462–7466 (1992).
Sackin, H. in Molecular Biology of Membrane Transport Disorders (ed. Schultz, S. G.) 201–222 (Plenum, New York, 1996).
Kolega, J. Effects of mechanical tension on protrusive activity and microfilament and intermediate filament organization in an epidermal epithelium moving in culture. J. Cell Biol. 102, 1400–1411 (1986).
Pommerenke, H.et al. Stimulation of integrin receptors using a magnetic drag force device induces an intracellular free calcium response. Eur. J. Cell Biol. 70, 157–164 (1996).
Glogauer, M., Ferrier, J. & McCulloch, C. A. G. Magnetic fields applied to collagen-coated ferric oxide beads induce stretch-activated Ca2+ flux in human fibroblasts. Am. J. Physiol. 269, C1093–C1104 (1995).
Naruse, K. & Sokabe, M. Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am. J. Physiol. 264, 1037–1044 (1993).
Chen, W.-T. Mechanism of retraction of the trailing edge during fibroblast movement. J. Cell Biol. 90, 187–200 (1981).
Hendey, B., Klee, C. B. & Maxfield, F. R. Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science 258, 296–299 (1992).
Huttenlocher, A.et al. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 272, 32719–32722 (1997).
Palecek, S. P., Huttenlocher, A., Horwitz, A. F. & Lauffenburger, D. A. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J. Cell Sci. 111, 929–940 (1998).
Brundage, R. A., Fogarty, K. E., Tuft, R. A. & Fay, F. S. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science 254, 703–706 (1991).
Marks, P. W. & Maxfield, F. R. Transient increases in cytosolic free calcium appear to be required for the migration of adherent human neutrophils. J. Cell Biol. 110, 43–52 (1990).
Ishihara, A., Gee, K., Schwartz, S., Jacobson, K. & Lee, J. Photoactivation of caged compounds in single, living cells: An application to the study of cell locomotion. Biotechniques 23, 268–274 (1997).
Acknowledgements
This work was supported by the NIH (K.J. and G.O.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J., Ishihara, A., Oxford, G. et al. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 400, 382–386 (1999). https://doi.org/10.1038/22578
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/22578
This article is cited by
-
The role of calcium oscillations in the phenotype selection in endothelial cells
Scientific Reports (2021)
-
The effects of valve leaflet mechanics on lymphatic pumping assessed using numerical simulations
Scientific Reports (2019)
-
Myosin-II mediated traction forces evoke localized Piezo1-dependent Ca2+ flickers
Communications Biology (2019)
-
Manipulation of cell migration by laserporation-induced local wounding
Scientific Reports (2019)
-
A temporal examination of calcium signaling in cancer- from tumorigenesis, to immune evasion, and metastasis
Cell & Bioscience (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.