Abstract
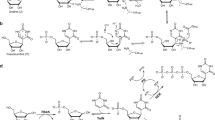
NUCLEOPHILIC substitution by semicarbazide in cytidine and its derivatives has been reported1. Such alterations of nucleic acid bases is an essential preliminary to the formation of synthetic “genetic messengers” to serve in the chemotherapy of growth abnormalities. Generally, methods for the synthesis of derivatives of nucleosides are of wide applicability to genetic problems. There is particular chemical interest in the reactions of dinitrophenyl hydrazine with nucleosides because the reagent replaces, in a specific manner, amino groups without respect to their position in a pyrimidine or purine ring.
Similar content being viewed by others
Article PDF
References
Hayatsu, H., Takeishi, K., and Ukita, T., Biochim. Biophys. Acta, 123, 445 (1966).
Gilham, P. T., J. Amer. Chem. Soc., 84, 687 (1962).
Michelson, A. M., The Chemistry of Nucleosides and Nucleotides, 20 (Academic Press, London, 1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
PATEL, A., BROWN, H. Selective Deamination of Nucleosides by 2,4-Dinitrophenyl Hydrazine. Nature 214, 402 (1967). https://doi.org/10.1038/214402a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/214402a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.