Abstract
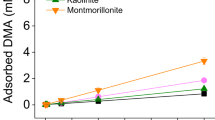
THE potassium ion fixed into the interlayer spacing of a clay mineral is not easily replaced, and the results of chemical analysis cannot distinguish these interlayer potassium and the structural potassium. Therefore, the total potassium obtained in the chemical analysis includes the gross structural potassium and the edge-site exchangeable potassium. One may assume, therefore, for the comparison of the adsorbability of various clay minerals, that the smaller the ratio of structural potassium, or alternatively the larger the ratio of exchangeable potassium to the total potassium, the greater will be the adsorption of metal ions, especially of rubidium and caesium, which are adsorbed in the dehydrated states having rather small cationic radii and can easily penetrate into the interlayer opening.
Similar content being viewed by others
Article PDF
References
Grim, R. E., Clay Mineralogy (McGraw Hill, 1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CHENG, HS. Role of Exchangeable Potassium and Magnesium on Caesium Absorption on Marine Sediments. Nature 207, 1010 (1965). https://doi.org/10.1038/2071010a0
Issue Date:
DOI: https://doi.org/10.1038/2071010a0
This article is cited by
-
The spatial distribution of physicochemical parameters in coastal sediments along the Bay of Bengal Coastal Zone with statistical analysis
Environmental Monitoring and Assessment (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.