Abstract
Clay minerals retain or adsorb metal ions in the Earth’s critical zone. Rocks, sediments and soils rich in clay minerals can concentrate rare earth elements (REEs) in ion adsorption-type deposits (IADs) and are similarly effective at metallic contaminant remediation. However, the molecular-scale chemical and physical mechanisms of metal ion retention remain only partly understood. In this Review, we describe the nature, location and energy requirements of metal retention at clay mineral surfaces. Retention originates mainly from electrostatic interactions during cation exchange at low pH and chemical bonding in surface complexation and precipitation at neutral and high pH. Surface complexation can induce surface redox reactions and precipitation mechanisms including neoformation of clay mineral layered structures. In IADs, outer-sphere adsorption is the major retention mechanism of REE ions. By contrast, the use of clay minerals in pollution control relies on various mechanisms that can coexist, including cation exchange, surface complexation and nucleation growth. To more effectively leverage clay mineral–metal interactions in resource recovery and contaminant remediation, complex mechanisms such as surface precipitation and redox reactions must be better understood; for instance, by utilizing advances in quantum mechanical calculations, close combination between synchrotron and simulation techniques, and upscaling of molecular-level information in macroscopic thermokinetic predictive models.
Key points
-
Clay minerals have a diverse array of chemical structures and layer types that lead to a range of metal ion retention mechanisms in Earth’s critical zone. The metal retention capabilities of clay minerals can concentrate rare earth elements (REEs) in ion adsorption-type deposits (IADs) and can also be exploited for metallic industrial waste disposal.
-
Basic metal ion–clay mineral interaction mechanisms include cation exchange, surface complexation, ligand exchange, structural incorporation, surface precipitation (with or without epitaxial growth of neoformed minerals) and precipitation induced by surface redox reactions.
-
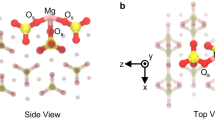
Such diversity of retention mechanisms originates from the distinct structures and properties of basal and edge surfaces. Cation exchange on basal surfaces occurs mainly through electrostatics whereas other mechanisms occur through chemical bonding on edge surfaces.
-
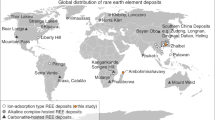
REEs in IADs are mainly physically adsorbed on basal surfaces, which are responsible for the high REE extractability (>50%) through ion exchange.
-
Both cation exchange and surface complexation processes occur during the retardation of metallic pollution plumes in waste management applications (radioactive and conventional industrial wastes as well as landfill leachate).
-
Understanding and quantification of the multifaceted and multiscale nature of clay mineral–metal ion interactions necessitate the close combination of experimental and modelling techniques at the molecular level.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bergaya, F. & Lagaly, G. Handbook of Clay Science 2nd edn (Elsevier, 2013).
Sposito, G., Skipper, N. T., Sutton, R., Park, S. & Soper, A. K. Surface geochemistry of the clay minerals. Proc. Natl Acad. Sci. USA 96, 3358–3364 (1999).
Li, M. Y. H. & Zhou, M.-F. The role of clay minerals in formation of the regolith-hosted heavy rare earth element deposits. Am. Min. 105, 92–108 (2020).
Delay, J., Distinguin, M. & Dewonck, S. Characterization of a clay-rich rock through development and installation of specific hydrogeological and diffusion test equipment in deep boreholes. Phys. Chem. Earth 32, 393–407 (2007).
Mishra, H., Karmakar, S., Kumar, R. & Kadambala, P. A long-term comparative assessment of human health risk to leachate-contaminated groundwater from heavy metal with different liner systems. Environ. Sci. Pollut. Res. 25, 2911–2923 (2018).
Yi, X. et al. Remediation of heavy metal-polluted agricultural soils using clay minerals: a review. Pedosphere 27, 193–204 (2017).
Brigatti, M. F., Galán, E. & Theng, B. K. G. Chapter 2 — structure and mineralogy of clay minerals. Handb. Clay Sci. 5, 21–81 (2013).
Yuan, G. D., Theng, B. K. G., Churchman, G. J. & Gates, W. P. Chapter 5.1 — clays and clay minerals for pollution control. Handb. Clay Sci. 5, 587–644 (2013).
Altmann, S. Geo’chemical research: a key building block for nuclear waste disposal safety cases. J. Contam. Hydrol. 102, 174–179 (2008).
Skipper, N., Soper, A. & McConnell, J. The structure of interlayer water in vermiculite. J. Chem. Phys. 94, 5751–5760 (1991).
Schlegel, M. L. et al. Cation sorption on the muscovite (0 0 1) surface in chloride solutions using high-resolution X-ray reflectivity. Geochim. Cosmochim. Acta 70, 3549–3565 (2006).
Dähn, R. et al. Neoformation of Ni phyllosilicate upon Ni uptake on montmorillonite: a kinetics study by powder and polarized extended X-ray absorption fine structure spectroscopy. Geochim. Cosmochim. Acta 66, 2335–2347 (2002).
Manceau, A. & Calas, G. Nickel-bearing clay minerals: II. Intracrystalline distribution of nickel: an X-ray absorption study. Clay Min. 21, 341–360 (1986).
Churakov, S. V. & Prasianakis, N. I. Review of the current status and challenges for a holistic process-based description of mass transport and mineral reactivity in porous media. Am. J. Sci. 318, 921–948 (2018).
Churakov, S. V. & Gimmi, T. Up-scaling of molecular diffusion coefficients in clays: a two-step approach. J. Phys. Chem. C. 115, 6703–6714 (2011).
Brouwer, E., Baeyens, B., Maes, A. & Cremers, A. Cesium and rubidium ion equilibria in illite clay. J. Phys. Chem. 87, 1213–1219 (1983).
Comans, R. N. J., Haller, M. & De Preter, P. Sorption of cesium on illite: non-equilibrium behaviour and reversibility. Geochim. Cosmochim. Acta 55, 433–440 (1991).
Oscarson, D. W., Hume, H. B. & King, F. Sorption of cesium on compacted bentonite. Clays Clay Miner. 42, 731–736 (1994).
De Koning, A. & Comans, R. N. J. Reversibility of radiocaesium sorption on illite. Geochim. Cosmochim. Acta 68, 2815–2823 (2004).
Poinssot, C., Baeyens, B. & Bradbury, M. H. Experimental studies of Cs, Sr, Ni, and Eu Sorption on Na-illite and the Modelling of Cs Sorption (Paul Scherrer Institut, 1999).
Verburg, K. & Baveye, P. Hysteresis in the binary exchange of cations on 2/1 clay-minerals — a critical-review. Clays Clay Miner. 42, 207–220 (1994).
Bellenger, J. P. & Staunton, S. Adsorption and desorption of Sr-85 and Cs-137 on reference minerals, with and without inorganic and organic surface coatings. J. Environ. Radioact. 99, 831–840 (2008).
Dyer, A., Chow, J. K. & Umar, I. M. The uptake of caesium and strontium radioisotopes onto clays. J. Mater. Chem. 10, 2734–2740 (2000).
Baeyens, B. & Bradbury, M. H. A mechanistic description of Ni and Zn sorption on Na-montmorillonite. Part I: titration and sorption measurements. J. Contam. Hydrol. 27, 199–222 (1997).
Gu, X. & Evans, L. J. Modelling the adsorption of Cd(II), Cu(II), Ni(II), Pb(II), and Zn(II) onto Fithian illite. J. Colloid Interface Sci. 307, 317–325 (2007).
Bradbury, M. H. & Baeyens, B. Sorption modelling on illite Part I: titration measurements and the sorption of Ni, Co, Eu and Sn. Geochim. Cosmochim. Acta 73, 990–1003 (2009).
Bradbury, M. H. & Baeyens, B. Modelling the sorption of Mn(II), Co(II), Ni(II), Zn(II), Cd(II), Eu(III), Am(III), Sn(IV), Th(IV), Np(V) and U(VI) on montmorillonite: linear free energy relationships and estimates of surface binding constants for some selected heavy metals and actinides. Geochim. Cosmochim. Acta 69, 875–892 (2005).
Akafia, M. M., Reich, T. J. & Koretsky, C. M. Assessing Cd, Co, Cu, Ni, and Pb sorption on montmorillonite using surface complexation models. Appl. Geochem. 26, S154–S157 (2011).
Marques Fernandes, M., Scheinost, A. & Baeyens, B. Sorption of trivalent lanthanides and actinides onto montmorillonite: macroscopic, thermodynamic and structural evidence for ternary hydroxo and carbonato surface complexes on multiple sorption sites. Water Res. 99, 74–82 (2016).
Bradbury, M. H. & Baeyens, B. Sorption modelling on illite. Part II: actinide sorption and linear free energy relationships. Geochim. Cosmochim. Acta 73, 1004–1013 (2009).
Bradbury, M. H. & Baeyens, B. Experimental measurements and modeling of sorption competition on montmorillonite. Geochim. Cosmochim. Acta 69, 4187–4197 (2005).
Grangeon, S. et al. The influence of natural trace element distribution on the mobility of radionuclides. The exemple of nickel in a clay-rock. Appl. Geochem. 52, 155–173 (2015).
Marques Fernandes, M. & Baeyens, B. Cation exchange and surface complexation of lead on montmorillonite and illite including competitive adsorption effects. Appl. Geochem. 100, 190–202 (2019).
Gao, Y., Shao, Z. & Xiao, Z. U(VI) sorption on illite: effect of pH, ionic strength, humic acid and temperature. J. Radioanal. Nucl. Chem. 303, 867–876 (2015).
Uddin, M. K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 308, 438–462 (2017).
Goldberg, S. & Glaubig, R. A. Anion sorption on a calcareous, montmorillonitic soil — selenium. Soil. Sci. Soc. Am. J. 52, 954–958 (1988).
Palmer, D. A. & Meyer, R. E. Adsorption of technetium on selected inorganic ion-exchange materials and on a range of naturally occurring minerals under oxic conditions. J. Inorg. Nucl. Chem. 43, 2979–2984 (1981).
Missana, T., Alonso, U. & Garcıa-Gutiérrez, M. Experimental study and modelling of selenite sorption onto illite and smectite clays. J. Colloid Interface Sci. 334, 132–138 (2009).
Peak, D., Saha, U. & Huang, P. Selenite adsorption mechanisms on pure and coated montmorillonite: an EXAFS and XANES spectroscopic study. Soil Sci. Soc. Am. J. 70, 192–203 (2006).
Ervanne, H., Hakanen, M. & Lehto, J. Selenium sorption on clays in synthetic groundwaters representing crystalline bedrock conditions. J. Radioanal. Nucl. Chem. 307, 1365–1373 (2016).
Goldberg, S. Modeling selenite adsorption envelopes on oxides, clay minerals, and soils using the triple layer model. Soil Sci. Soc. Am. J. 77, 64–71 (2013).
Manning, B. A. & Goldberg, S. Adsorption and stability of arsenic(III) at the clay mineral–water interface. Environ. Sci. Technol. 31, 2005–2011 (1997).
Garcia-Sanchez, A., Alvarez-Ayuso, E. & Rodriguez-Martin, F. Sorption of As(V) by some oxyhydroxides and clay minerals. Application to its immobilization in two polluted mining soils. Clay Miner. 37, 187–194 (2002).
Mohapatra, D., Mishra, D., Chaudhury, G. R. & Das, R. Arsenic (V) adsorption mechanism using kaolinite, montmorillonite and illite from aqueous medium. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 42, 463–469 (2007).
Goldberg, S. Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci. Soc. Am. J. 66, 413–421 (2002).
Xi, J., He, M. & Lin, C. Adsorption of antimony (III) and antimony (V) on bentonite: kinetics, thermodynamics and anion competition. Microchem. J. 97, 85–91 (2011).
Goldberg, S., Forster, H. & Godfrey, C. Molybdenum adsorption on oxides, clay minerals, and soils. Soil. Sci. Soc. Am. J. 60, 425–432 (1996).
Lee, S. M. & Tiwari, D. Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: an overview. Appl. Clay Sci. 59, 84–102 (2012).
Fletcher, P. & Sposito, G. The chemical modeling of clay/electrolyte interactions for montmorillonite. Clay Miner. 24, 375–391 (1989).
Bradbury, M. H. & Baeyens, B. A mechanistic description of Ni and Zn sorption on Na-montmorillonite. Part II: modeling. J. Contam. Hydrol. 27, 223–248 (1997).
Loganathan, N. & Kalinichev, A. G. Quantifying the mechanisms of site-specific ion exchange at an inhomogeneously charged surface: case of Cs+/K+ on hydrated muscovite mica. J. Phys. Chem. C 121, 7829–7836 (2017).
Loganathan, N., Yazaydin, A. O., Bowers, G. M., Kalinichev, A. G. & Kirkpatrick, R. J. Structure, energetics, and dynamics of Cs+ and H2O in hectorite: molecular dynamics simulations with an unconstrained substrate surface. J. Phys. Chem. C. 120, 10298–10310 (2016).
Liu, X., Lu, X., Wang, R. & Zhou, H. Effects of layer-charge distribution on the thermodynamic and microscopic properties of Cs-smectite. Geochim. Cosmochim. Acta 72, 1837–1847 (2008).
Tournassat, C., Grangeon, S., Leroy, P. & Giffaut, E. Modeling specific pH dependent sorption of divalent metals on montmorillonite surfaces. A review of pitfalls, recent achievements and current challenges. Am. J. Sci. 313, 395–451 (2013).
Thomas, H. C. & Gaines, G. L. J. The thermodynamics of ion exchange on clay minerals. A preliminary report on the system montmorillonite–Cs–Sr. Clays Clay Miner. 2, 398–403 (1953).
Vanselow, A. P. The utilization of the base-exchange reaction for the determination of activity coefficients in mixed electrolytes. J. Am. Chem. Soc. 54, 1307–1311 (1932).
Bourg, I. C. & Sposito, G. Ion Exchange Phenomena (OSTI, 2011).
Tournassat, C. et al. Cation exchange selectivity coefficient values on smectite and mixed-layer illite/smectite minerals. Soil. Sci. Soc. Am. J. 73, 928–942 (2009).
Lammers, L. N. et al. Molecular dynamics simulations of cesium adsorption on illite nanoparticles. J. Colloid Interface Sci. 490, 608–620 (2016).
Poinssot, C., Baeyens, B. & Bradbury, M. H. Experimental and modelling studies of caesium sorption on illite. Geochim. Cosmochim. Acta 63, 3217–3227 (1999).
Bergaoui, L., Lambert, J. F. & Prost, R. Cesium adsorption on soil clay: macroscopic and spectroscopic measurements. Appl. Clay Sci. 29, 23–29 (2005).
Meleshyn, A. Adsorption of Sr2+ and Ba2+ at the cleaved mica–water interface: free energy profiles and interfacial structure. Geochim. Cosmochim. Acta 74, 1485–1497 (2010).
Bourg, I. C., Lee, S. S., Fenter, P. & Tournassat, C. Stern layer structure and energetics at mica–water interfaces. J. Phys. Chem. C. 121, 9402–9412 (2017).
Zaunbrecher, L. K., Cygan, R. T. & Elliott, W. C. Molecular models of cesium and rubidium adsorption on weathered micaceous minerals. J. Phys. Chem. A 119, 5691–5700 (2015).
Rotenberg, B., Marry, V., Malikova, N. & Turq, P. Molecular simulation of aqueous solutions at clay surfaces. J. Phys. Condens. Matter 22, 284114 (2010).
Tournassat, C., Chapron, Y., Leroy, P. & Boulahya, F. Comparison of molecular dynamics simulations with triple layer and modified Gouy–Chapman models in a 0.1 M NaCl–montmorillonite system. J. Colloid Interface Sci. 339, 533–541 (2009).
Li, X., Liu, N. & Zhang, J. Adsorption of cesium at the external surface of TOT type clay mineral: effect of the interlayer cation and the hydrated state. J. Phys. Chem. C 123, 19540–19548 (2019).
Tournassat, C., Bourg, I. C., Steefel, C. I. & Bergaya, F. Chapter 1 — surface properties of clay minerals. Nat. Engin. Clay Barriers 6, 5–31 (2015).
Liu, L. Prediction of swelling pressures of different types of bentonite in dilute solutions. Colloids Surf. A Physicochem. Eng. Asp. 434, 303–318 (2013).
Van Loon, L. R. & Glaus, M. A. Mechanical compaction of smectite clays increases ion exchange selectivity for cesium. Environ. Sci. Technol. 42, 1600–1604 (2008).
Rotenberg, B., Morel, J.-P., Marry, V., Turq, P. & Morel-Desrosiers, N. On the driving force of cation exchange in clays: insights from combined microcalorimetry experiments and molecular simulation. Geochim. Cosmochim. Acta 73, 4034–4044 (2009).
Teppen, B. J. & Miller, D. M. Hydration energy determines isovalent cation exchange selectivity by clay minerals. Soil. Sci. Soc. Am. J. 70, 31–40 (2006).
Cygan, R. T., Greathouse, J. A. & Kalinichev, A. G. Advances in Clayff molecular simulation of layered and nanoporous materials and their aqueous interfaces. J. Phys. Chem. C 125, 17573–17589 (2021).
Le Crom, S., Tournassat, C., Robinet, J.-C. & Marry, V. Influence of polarisability on the prediction of the electrical double layer structure in a clay mesopore: a molecular dynamics study. J. Phys. Chem. C 124, 6221–6232 (2020).
Tesson, S. et al. Classical polarizable force field to study hydrated charged clays and zeolites. J. Phys. Chem. C 122, 24690–24704 (2018).
Churakov, S. V. Mobility of Na and Cs on montmorillonite surface under partially saturated conditions. Environ. Sci. Technol. 47, 9816–9823 (2013).
Simonnin, P., Marry, V., Noetinger, B., Nieto-Draghi, C. & Rotenberg, B. Mineral-and ion-specific effects at clay–water interfaces: structure, diffusion, and hydrodynamics. J. Phys. Chem. C 122, 18484–18492 (2018).
Malikova, N., Dubois, E., Marry, V., Rotenberg, B. & Turq, P. Dynamics in clays — combining neutron scattering and microscopic simulation. Z. Phys. Chem. 224, 153–181 (2010).
Porion, P. et al. 133Cs nuclear magnetic resonance relaxometry as a probe of the mobility of cesium cations confined within dense clay sediments. J. Phys. Chem. C 119, 15360–15372 (2015).
Appelo, C. A. J., Van Loon, L. R. & Wersin, P. Multicomponent diffusion of a suite of tracers (HTO, Cl, Br, I, Na, Sr, Cs) in a single sample of Opalinus clay. Geochim.Cosmochim. Acta 74, 1201–1219 (2010).
Churakov, S. V., Gimmi, T., Unruh, T., Van Loon, L. R. & Juranyi, F. Resolving diffusion in clay minerals at different time scales: combination of experimental and modeling approaches. Appl. Clay Sci. 96, 36–44 (2014).
Tournassat, C. & Steefel, C. I. Reactive transport modeling of coupled processes in nanoporous media. Rev. Mineral. Geochem. 85, 75–110 (2019).
Altmann, S. et al. Diffusion-driven transport in clayrock formations. Appl. Geochem. 27, 463–478 (2012).
Charlet, L., Alt-Epping, P., Wersin, P. & Gilbert, B. Diffusive transport and reaction in clay rocks: a storage (nuclear waste, CO2, H2), energy (shale gas) and water quality issue. Adv. Water Resour. 106, 39–59 (2017).
Grambow, B. Geological disposal of radioactive waste in clay. Elements 12, 239–245 (2016).
Whittaker, M. L., Lammers, L. N., Carrero, S., Gilbert, B. & Banfield, J. F. Ion exchange selectivity in clay is controlled by nanoscale chemical–mechanical coupling. Proc. Natl Acad. Sci. USA 116, 22052–22057 (2019).
Liu, X., Cheng, J., Sprik, M., Lu, X. & Wang, R. Interfacial structures and acidity of edge surfaces of ferruginous smectites. Geochim. Cosmochim. Acta 168, 293–301 (2015).
Liu, X., Cheng, J., Sprik, M., Lu, X. & Wang, R. Surface acidity of 2:1-type dioctahedral clay minerals from first principles molecular dynamics simulations. Geochim.Cosmochim. Acta 140, 410–417 (2014).
Liu, X. et al. Acidity of edge surface sites of montmorillonite and kaolinite. Geochim. Cosmochim. Acta 117, 180–190 (2013).
Genuchten, C. Mvan & Peña, J. Antimonate and arsenate speciation on reactive soil minerals studied by differential pair distribution function analysis. Chem. Geol. 429, 1–9 (2016).
Dähn, R. et al. Structural evidence for the sorption of Ni(II) atoms on the edges of montmorillonite clay minerals: a polarized X-ray absorption fine structure study. Geochim. Cosmochim. Acta 37, 1–15 (2003).
Dähn, R., Baeyens, B. & Bradbury, M. H. Investigation of the different binding edge sites for Zn on montmorillonite using P-EXAFS — the strong/weak site concept in the 2SPNE SC/CE sorption model. Geochim. Cosmochim. Acta 75, 5154–5168 (2011).
Churakov, S. V. & Dähn, R. Zinc adsorption on clays inferred from atomistic simulations and EXAFS spectroscopy. Environ. Sci. Technol. 46, 5713–5719 (2012).
Rabung, T. et al. Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 1: batch sorption and time-resolved laser fluorescence spectroscopy experiments. Geochim. Cosmochim. Acta 69, 5393–5402 (2005).
Sasaki, T. et al. Sorption of Eu3+ on Na-montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modeling. J. Nucl. Sci. Technol. 53, 592–601 (2016).
Verma, P. K. et al. Eu (III) sorption onto various montmorillonites: experiments and modeling. Appl. Clay Sci. 175, 22–29 (2019).
Zhang, C. et al. Cadmium (II) complexes adsorbed on clay edge surfaces: insight from first principles molecular dynamics simulation. Clays Clay Miner. 64, 337–347 (2016).
Zhang, C. et al. Surface complexation of heavy metal cations on clay edges: insights from first principles molecular dynamics simulation of Ni(II). Geochim. Cosmochim. Acta 203, 54–68 (2017).
Tournassat, C., Tinnacher, R. M., Grangeon, S. & Davis, J. A. Modeling uranium (VI) adsorption onto montmorillonite under varying carbonate concentrations: a surface complexation model accounting for the spillover effect on surface potential. Geochim. Cosmochim. Acta 220, 291–308 (2018).
Marques Fernandes, M., Baeyens, B., Dähn, R., Scheinost, A. & Bradbury, M. U(VI) sorption on montmorillonite in the absence and presence of carbonate: a macroscopic and microscopic study. Geochim. Cosmochim. Acta 93, 262–277 (2012).
Kremleva, A., Martorell, B., Krüger, S. & Rösch, N. Uranyl adsorption on solvated edge surfaces of pyrophyllite: a DFT model study. Phys. Chem. Chem. Phys. 14, 5815–5823 (2012).
Kremleva, A., Krüger, S. & Rösch, N. Uranyl adsorption at solvated edge surfaces of 2:1 smectites. A density functional study. Phys. Chem. Chem. Phys. 17, 13757–13768 (2015).
Kremleva, A., Krüger, S. & Rösch, N. Toward a reliable energetics of adsorption at solvated mineral surfaces: a computational study of uranyl (VI) on 2:1 clay minerals. J. Phys. Chem. C. 120, 324–335 (2016).
Zhang, C., Liu, X., Tinnacher, R. M. & Tournassat, C. Mechanistic understanding of uranyl ion complexation on montmorillonite edges: a combined first-principles molecular dynamics–surface complexation modeling approach. Environ. Sci. Technol. 52, 8501–8509 (2018).
Catalano, J. G. & Brown, G. E. Jr. Uranyl adsorption onto montmorillonite: evaluation of binding sites and carbonate complexation. Geochim. Cosmochim. Acta 69, 2995–3005 (2005).
Schlegel, M. L. & Descostes, M. Uranium uptake by hectorite and montmorillonite: a solution chemistry and polarized EXAFS study. Environ. Sci. Technol. 43, 8593–8598 (2009).
Ford, R. G. & Sparks, D. L. The nature of Zn precipitates formed in the presence of pyrophyllite. Environ. Sci. Technol. 34, 2479–2483 (2000).
Schlegel, M. L. & Manceau, A. Evidence for the nucleation and epitaxial growth of Zn phyllosilicate on montmorillonite. Geochim. Cosmochim. Acta 70, 901–917 (2006).
Scheidegger, A. M., Lamble, G. M. & Spark, D. L. Spectroscopic evidence for the formation of mixed-cation hydroxide phases upon metal sorption on clays and aluminum oxides. J. Colloid Interface Sci. 186, 118–128 (1997).
Siebecker, M., Li, W., Khalid, S. & Sparks, D. Real-time QEXAFS spectroscopy measures rapid precipitate formation at the mineral–water interface. Nat. Commun. 5, 5003 (2014).
Thompson, H. A., Parks, G. A. & BrownJr, G. E. Dynamic interactions of dissolution, surface adsorption, and precipitation in an aging cobalt(II)–clay–water system. Geochim. Cosmochim. Acta 63, 1767–1779 (1999).
Starcher, A. N., Li, W., Kukkadapu, R. K., Elzinga, E. J. & Sparks, D. L. Fe(II) sorption on pyrophyllite: effect of structural Fe (III)(impurity) in pyrophyllite on nature of layered double hydroxide (LDH) secondary mineral formation. Chem. Geol. 439, 152–160 (2016).
Zhu, Y. & Elzinga, E. J. Formation of layered Fe(II)-hydroxides during Fe(II) sorption onto clay and metal-oxide substrates. Environ. Sci. Technol. 48, 4937–4945 (2014).
Zhang, C., Liu, X., Lu, X., Meijer, E. J. & Wang, R. Understanding the heterogeneous nucleation of heavy metal phyllosilicates on clay edges with first-principles molecular dynamics. Environ. Sci. Technol. 53, 13704–13712 (2019).
Jacquat, O., Voegelin, A., Villard, A., Marcus, M. A. & Kretzschmar, R. Formation of Zn-rich phyllosilicate, Zn-layered double hydroxide and hydrozincite in contaminated calcareous soils. Geochim. Cosmochim. Acta 72, 5037–5054 (2008).
Choulet, F., Buatier, M., Barbanson, L., Guégan, R. & Ennaciri, A. Zinc-rich clays in supergene non-sulfide zinc deposits. Miner. Depos. 51, 467–490 (2016).
Roqué-Rosell, J., Villanova-de-Benavent, C. & Proenza, J. A. The accumulation of Ni in serpentines and garnierites from the Falcondo Ni-laterite deposit (Dominican Republic) elucidated by means of μXAS. Geochim. Cosmochim. Acta 198, 48–69 (2017).
Balassone, G., Nieto, F., Arfè, G., Boni, M. & Mondillo, N. Zn-clay minerals in the Skorpion Zn nonsulfide deposit (Namibia): identification and genetic clues revealed by HRTEM and AEM study. Appl. Clay Sci. 150, 309–322 (2017).
Chassé, M., Griffin, W. L., O’Reilly, S. Y. & Calas, G. Australian laterites reveal mechanisms governing scandium dynamics in the critical zone. Geochim. Cosmochim. Acta 260, 292–310 (2019).
Stucki, J. W. Chapter 11 — properties and behaviour of iron in clay minerals. Handb. Clay Sci. 5, 559–611 (2013).
Huang, J. et al. Fe(II) redox chemistry in the environment. Chem. Rev. 121, 8161–8233 (2021).
Chakraborty, S. et al. U(VI) sorption and reduction by Fe(II) sorbed on montmorillonite. Environ. Sci. Technol. 44, 3779–3785 (2010).
Liger, E., Charlet, L. & Van Cappellen, P. Surface catalysis of uranium (VI) reduction by iron (II). Geochim. Cosmochim. Acta 63, 2939–2955 (1999).
Brookshaw, D. R. et al. Redox interactions of Tc(VII), U(VI), and Np(V) with microbially reduced biotite and chlorite. Environ. Sci. Technol. 49, 13139–13148 (2015).
Begg, J. D., Edelman, C., Zavarin, M. & Kersting, A. B. Sorption kinetics of plutonium (V)/(VI) to three montmorillonite clays. Appl. Geochem. 96, 131–137 (2018).
Hixon, A. E. & Powell, B. A. Plutonium environmental chemistry: mechanisms for the surface-mediated reduction of Pu(V/VI). Environ. Sci. Process. Impacts 20, 1306–1322 (2018).
Charlet, L. et al. Electron transfer at the mineral/water interface: selenium reduction by ferrous iron sorbed on clay. Geochim. Cosmochim. Acta 71, 5731–5749 (2007).
Ilgen, A. G., Kruichak, J. N., Artyushkova, K., Newville, M. G. & Sun, C. Redox transformations of As and Se at the surfaces of natural and synthetic ferric nontronites: role of structural and adsorbed Fe(II). Environ. Sci. Technol. 51, 11105–11114 (2017).
Bishop, M. E., Dong, H., Kukkadapu, R. K., Liu, C. & Edelmann, R. E. Bioreduction of Fe-bearing clay minerals and their reactivity toward pertechnetate (Tc-99). Geochim. Cosmochim. Acta 75, 5229–5246 (2011).
Jaisi, D. P. et al. Reduction and long-term immobilization of technetium by Fe(II) associated with clay mineral nontronite. Chem. Geol. 264, 127–138 (2009).
Qafoku, O. et al. Tc(VII) and Cr(VI) interaction with naturally reduced ferruginous smectite from a redox transition zone. Environ. Sci. Technol. 51, 9042–9052 (2017).
Brigatti, M. F. et al. Reduction and sorption of chromium by Fe(II)-bearing phyllosilicates: chemical treatments and X-ray absorption spectroscopy (XAS) studies. Clays Clay Miner. 48, 272–281 (2000).
Joe-Wong, C., Brown, G. E. Jr & Maher, K. Kinetics and products of chromium (VI) reduction by iron (II/III)-bearing clay minerals. Environ. Sci. Technol. 51, 9817–9825 (2017).
Liao, W. et al. Effect of coexisting Fe (III)(oxyhydr)oxides on Cr(VI) reduction by Fe(II)-bearing clay minerals. Environ. Sci. Technol. 53, 13767–13775 (2019).
Bishop, M. E., Glasser, P., Dong, H., Arey, B. & Kovarik, L. Reduction and immobilization of hexavalent chromium by microbially reduced Fe-bearing clay minerals. Geochim. Cosmochim. Acta 133, 186–203 (2014).
Scheinost, A. C. et al. X-ray absorption and photoelectron spectroscopy investigation of selenite reduction by Fe-II-bearing minerals. J. Contam. Hydrol. 102, 228–245 (2008).
Ilgen, A. G., Foster, A. L. & Trainor, T. P. Role of structural Fe in nontronite NAu-1 and dissolved Fe(II) in redox transformations of arsenic and antimony. Geochim. Cosmochim. Acta 94, 128–145 (2012).
Cheng, J. & Sprik, M. Alignment of electronic energy levels at electrochemical interfaces. Phys. Chem. Chem. Phys. 14, 11245–11267 (2012).
Blumberger, J. Recent advances in the theory and molecular simulation of biological electron transfer reactions. Chem. Rev. 115, 11191–11238 (2015).
Cheng, J., Liu, X., Kattirtzi, J. A., VandeVondele, J. & Sprik, M. Aligning electronic and protonic energy levels of proton-coupled electron transfer in water oxidation on aqueous TiO2. Angew. Chem. 126, 12242–12246 (2014).
Anisimov, V. I., Zaanen, J. & Andersen, O. K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 44, 943 (1991).
Behler, J., Delley, B., Reuter, K. & Scheffler, M. Nonadiabatic potential-energy surfaces by constrained density-functional theory. Phys. Rev. B 75, 115409 (2007).
Alexandrov, V. & Rosso, K. M. Insights into the mechanism of Fe(II) adsorption and oxidation at Fe–clay mineral surfaces from first-principles calculations. J. Phys. Chem. C. 117, 22880–22886 (2013).
Liu, X., Cheng, J. & Sprik, M. Aqueous transition-metal cations as impurities in a wide gap oxide: the Cu2+/Cu+ and Ag2+/Ag+ redox couples revisited. J. Phys. Chem. B 119, 1152–1163 (2015).
Cheng, J. & VandeVondele, J. Calculation of electrochemical energy levels in water using the random phase approximation and a double hybrid functional. Phys. Rev. Lett. 116, 086402 (2016).
Cheng, J., Liu, X., VandeVondele, J., Sulpizi, M. & Sprik, M. Redox potentials and acidity constants from density functional theory based molecular dynamics. Acc. Chem. Res. 47, 3522–3529 (2014).
Ferrage, E. et al. Hydration properties and interlayer organization of water and ions in synthetic Na-smectite with tetrahedral layer charge. Part 2. Toward a precise coupling between molecular simulations and diffraction data. J. Phys. Chem. C. 115, 1867–1881 (2011).
Tambach, T. J., Hensen, E. J. M. & Smit, B. Molecular simulations of swelling clay minerals. J. Phys. Chem. B 108, 7586–7596 (2004).
Le Crom, S., Tournassat, C., Robinet, J.-C. & Marry, V. Influence of water saturation level on electrical double layer properties in a clay mineral mesopore: a molecular dynamics study. J. Phys. Chem. C 126, 647–654 (2022).
Savoye, S., Beaucaire, C., Fayette, A., Herbette, M. & Coelho, D. Mobility of cesium through the Callovo-Oxfordian claystones under partially saturated conditions. Environ. Sci. Technol. 46, 2633–2641 (2012).
Huang, B. et al. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal(loid)s in soil: a review. Environ. Sci. Process. Impacts 22, 1596–1615 (2020).
Kleber, M. et al. Mineral–organic associations: formation, properties, and relevance in soil environments. Adv. Agron. 130, 1–140 (2015).
Lagaly, G., Ogawa, M. & Dékény, I. Chapter 10.3 — clay mineral–organic interactions. Handb. Clay Sci. 5, 435–505 (2013).
Kleber, M. et al. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2, 402–421 (2021).
Sutton, R. & Sposito, G. Molecular structure in soil humic substances: the new view. Environ. Sci. Technol. 39, 9009–9015 (2005).
Piccolo, A. The supramolecular structure of humic substances. Soil. Sci. 166, 810–832 (2001).
Schnitzer, M. A lifetime perspective on the chemistry of soil organic matter. Adv. Agron. 68, 1–58 (1999).
Stevenson, F. J. Humus Chemistry: Genesis, Composition, Reactions (Wiley, 1994).
Colombo, C. et al. Spontaneous aggregation of humic acid observed with AFM at different pH. Chemosphere 138, 821–828 (2015).
Kelleher, B. P. & Simpson, A. J. Humic substances in soils: are they really chemically distinct? Environ. Sci. Technol. 40, 4605–4611 (2006).
Petrov, D., Tunega, D., Gerzabek, M. H. & Oostenbrink, C. Molecular dynamics simulations of the standard leonardite humic acid: microscopic analysis of the structure and dynamics. Environ. Sci. Technol. 51, 5414–5424 (2017).
Zhang, Y., Liu, X., Zhang, C. & Lu, X. A combined first principles and classical molecular dynamics study of clay-soil organic matters (SOMs) interactions. Geochim. Cosmochim. Acta 291, 110–125 (2020).
Willemsen, J. A., Myneni, S. C. & Bourg, I. C. Molecular dynamics simulations of the adsorption of phthalate esters on smectite clay surfaces. J. Phys. Chem. C. 123, 13624–13636 (2019).
Tournassat, C., Steefel, C., Bourg, I. & Bergaya, F. Natural and Engineered Clay Barriers (Elsevier, 2015).
Gates, W. P., Bouazza, A. & Churchman, G. J. Bentonite clay keeps pollutants at bay. Elements 5, 105–110 (2009).
Otunola, B. O. & Ololade, O. O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 18, 100692 (2020).
Delage, P., Cui, Y.-J. & Tang, A. M. Clays in radioactive waste disposal. J. Rock. Mech. Geotech. Eng. 2, 111–123 (2010).
Mukherjee, S. in The Science of Clays Ch. 21 309–325 (Springer, 2013).
Sellin, P. & Leupin, O. X. The use of clay as an engineered barrier in radioactive-waste management — a review. Clays Clay Miner. 61, 477–498 (2013).
Wypych, F., Bergaya, F. & Schoonheydt, R. A. From polymers to clay polymer nanocomposites. Dev. Clay Sci. 9, 331–359 (2018).
Bergaya, F. & Lagaly, G. Chapter 10.0 — introduction on modified clays and clay minerals. Handb. Clay Sci. 5, 383 (2013).
Vicente, M. A., Gil, A. & Bergaya, F. Chapter 10.5 — pillared clays and clay minerals. Handb. Clay Sci. 5, 523–557 (2013).
Dutta, D. K. Clay mineral catalysts. Dev. Clay Sci. 9, 289–329 (2018).
Bhowmick, S. et al. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: kinetics and mechanism. Chem. Eng. J. 243, 14–23 (2014).
Han, H. et al. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 369, 780–796 (2019).
Yadav, V. B., Gadi, R. & Kalra, S. Clay based nanocomposites for removal of heavy metals from water: a review. J. Environ. Manage. 232, 803–817 (2019).
Zhang, T. et al. Removal of heavy metals and dyes by clay-based adsorbents: from natural clays to 1D and 2D nano-composites. Chem. Eng. J. 420, 127574 (2020).
Buruga, K. et al. A review on functional polymer-clay based nanocomposite membranes for treatment of water. J. Hazard. Mater. 379, 120584 (2019).
Jlassi, K., Chehimi, M. M. & Thomas, S. Clay–Polymer Nanocomposites (Elsevier, 2017).
Payne, T. E. et al. Guidelines for thermodynamic sorption modelling in the context of radioactive waste disposal. Environ. Model. Softw. 42, 143–156 (2013).
Caporale, A. G. & Violante, A. Chemical processes affecting the mobility of heavy metals and metalloids in soil environments. Curr. Pollut. Rep. 2, 15–27 (2016).
Manceau, A. et al. Quantitative Zn speciation in smelter-contaminated soils by EXAFS spectroscopy. Am. J. Sci. 300, 289–343 (2000).
Appelo, C. A. J., Vinsot, A., Mettler, S. & Wechner, S. Obtaining the porewater composition of a clay rock by modeling the in- and out-diffusion of anions and cations from an in-situ experiment. J. Contam. Hydrol. 101, 67–76 (2008).
Appelo, C. A. J. & Wersin, P. Multicomponent diffusion modeling in clay systems with application to the diffusion of tritium, iodide, and sodium in Opalinus clay. Environ. Sci. Technol. 41, 5002–5007 (2007).
Soler, J. M., Steefel, C. I., Gimmi, T., Leupin, O. X. & Cloet, V. Modeling the ionic strength effect on diffusion in clay. The DR-A experiment at Mont Terri. ACS Earth Space Chem. 3, 442–451 (2019).
Gimmi, T. & Kosakowski, G. How mobile are sorbed cations in clays and clay rocks? Environ. Sci. Technol. 45, 1443–1449 (2011).
Glaus, M. et al. Cation diffusion in the electrical double layer enhances the mass transfer rates for Sr2+, Co2+ and Zn2+ in compacted illite. Geochim. Cosmochim. Acta 165, 376–388 (2015).
Glaus, M., Frick, S., & Van Loon, L. A coherent approach for cation surface diffusion in clay minerals and cation sorption models: diffusion of Cs+ and Eu3+ in compacted illite as case examples. Geochim. Cosmochim. Acta 274, 79–96 (2020).
Moldoveanu, G. & Papangelakis, V. An overview of rare-earth recovery by ion-exchange leaching from ion-adsorption clays of various origins. Mineral. Mag. 80, 63–76 (2016).
Sanematsu, K., Kon, Y., Imai, A., Watanabe, K. & Watanabe, Y. Geochemical and mineralogical characteristics of ion-adsorption type REE mineralization in Phuket, Thailand. Miner Deposita 48, 437–451 (2013).
Li, M. Y. H., Zhou, M.-F. & Williams-Jones, A. E. The genesis of regolith-hosted heavy rare earth element deposits: insights from the world-class Zudong deposit in Jiangxi Province, South China. Econ. Geol. 114, 541–568 (2019).
Borst, A. M. et al. Adsorption of rare earth elements in regolith-hosted clay deposits. Nat. Commun. 11, 4386 (2020).
Chi, R., Tian, J. Weathered Crust Elution-Deposited Rare Earth Ores (Nova Science, 2008).
Li, Y. H. M., Zhao, W. W. & Zhou, M.-F. Nature of parent rocks, mineralization styles and ore genesis of regolith-hosted REE deposits in South China: an integrated genetic model. J. Asian Earth Sci. 148, 65–95 (2017).
Goodenough, K. M., Wall, F. & Merriman, D. The rare earth elements: demand, global resources, and challenges for resourcing future generations. Nat. Resour. Res. 27, 201–216 (2018).
Jordens, A., Cheng, Y. P. & Waters, K. E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 41, 97–114 (2013).
Berger, A., Janots, E., Gnos, E., Frei, R. & Bernier, F. Rare earth element mineralogy and geochemistry in a laterite profile from Madagascar. Appl. Geochem. 41, 218–228 (2014).
Bern, C. R., Yesavage, T. & Foley, N. K. Ion-adsorption REEs in regolith of the Liberty Hill pluton, South Carolina, USA: an effect of hydrothermal alteration. J. Geochem. Explor. 172, 29–40 (2017).
Sanematsu, K. & Watanabe, Y. Characteristics and genesis of ion adsorption type REE deposits in the weathering crusts of metamorphic rocks in Ningdu, Ganzhou, China. Ore Geol. Rev. 135, 104173 (2016).
Yamaguchi, A., Honda, T., Tanaka, M., Tanaka, K. & Takahashi, Y. Discovery of ion-adsorption type deposits of rare earth elements (REE) in southwest Japan with speciation of REE by extended X-ray absorption fine structure spectroscopy. Geochem. J. 52, 415–425 (2018).
Braun, J.-J. et al. Cerium anomalies in lateritic profiles. Geochim. Cosmochim. Acta 54, 781–795 (1990).
Takahashi, Y., Shimizu, H., Usui, A., Kagi, H. & Nomura, M. Direct observation of tetravalent cerium in ferromanganese nodules and crusts by X-ray-absorption near-edge structure (XANES). Geochim. Cosmochim. Acta 64, 2929–2935 (2000).
Moldoveanu, G. A. & Papangelakis, V. G. Recovery of rare earth elements adsorbed on clay minerals: I. Desorption mechanism. Hydrometallurgy 117, 71–78 (2012).
Jones, D. J., Rozière, J., Olivera-Pastor, P., Rodrıguez-Castellòn, E. & Jimènez-Lòpez, A. Local environment of intercalated lanthanide ions in vermiculite. J. Chem. Soc. Faraday Trans. 87, 3077–3081 (1991).
Takahashi, Y., Kimura, T., Kato, Y., Minai, Y. & Tominaga, T. Characterization of Eu(III) species sorbed on silica and montmorillonite by laser-induced fluorescence spectroscopy. Radiochim. Acta 82, 227–232 (1998).
Stumpf, T., Bauer, A., Coppin, F., Fanghänel, T. & Kim, J.-I. Inner-sphere, outer-sphere and ternary surface complexes: a TRLFS study of the sorption process of Eu(III) onto smectite and kaolinite. Radiochim. Acta 90, 345–349 (2002).
Mukai, H., Kon, Y., Sanematsu, K., Takahashi, Y. & Ito, M. Microscopic analyses of weathered granite in ion-adsorption rare earth deposit of Jianxi Province, China. Sci. Rep. 10, 1–11 (2020).
Velde, B. B. & Meunier, A. The Origin of Clay Minerals in Soils and Weathered Rocks (Springer, 2008).
Nagasawa, M., Qin, H.-B., Yamaguchi, A. & Takahashi, Y. Local structure of rare earth elements (REE) in marine ferromanganese oxides by extended X-ray absorption fine structure and its comparison with REE in ion-adsorption type deposits. Chem. Lett. 49, 909–911 (2020).
Ohta, A., Kagi, H., Tsuno, H., Nomura, M. & Kawabe, I. Influence of multi-electron excitation on EXAFS spectroscopy of trivalent rare-earth ions and elucidation of change in hydration number through the series. Am. Mineral. 93, 1384–1392 (2008).
Stumpf, S. et al. Sorption of Am(III) onto 6-line-ferrihydrite and its alteration products: investigations by EXAFS. Environ. Sci. Technol. 40, 3522–3528 (2006).
Ohta, A., Kagi, H., Nomura, M., Tsuno, H. & Kawabe, I. Coordination study of rare earth elements on Fe oxyhydroxide and Mn dioxides: part II. Correspondence of structural change to irregular variations of partitioning coefficients and tetrad effect variations appearing in interatomic distances. Am. Mineral. 94, 476–486 (2009).
Kashiwabara, T. et al. Synchrotron X-ray spectroscopic perspective on the formation mechanism of REY-rich muds in the Pacific Ocean. Geochim. Cosmochim. Acta 240, 274–292 (2018).
Butt, C. R. & Cluzel, D. Nickel laterite ore deposits: weathered serpentinites. Elements 9, 123–128 (2013).
Bergaya, F. & Lagaly, G. Chapter 1 — general introduction: clays, clay minerals, and clay science. Handb. Clay Sci. A. Fund. 5, 1–19 (2013).
Schoonheydt, R. A., Johnston, C. T. & Bergaya, F. Clay minerals and their surfaces. Dev. Clay Sci. 9, 1–21 (2018).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133 (1965).
Marx, D. & Hutter, J. Ab Initio Molecular Dynamics: Basic Theory and Advanced Methods (Cambridge Univ. Press, 2009).
Frenkel, D. & Smit, B. Understanding Molecular Simulation: From Algorithms to Applications (Academic, 2002).
Liu, X., Tournassat, C. & Steefel, C. I. Preface to multiscale simulation in geochemistry. Geochim. Cosmochim. Acta 291, 1–4 (2020).
Sposito, G. The Surface chemistry of Natural Particles Vol. 242 (Oxford Univ. Press, 2004).
Steefel, C. I. et al. Reactive transport codes for subsurface environmental simulation. Comp. Geosci. 19, 445–478 (2015).
Steefel, C. I. Reactive transport at the crossroads. Rev. Mineral. Geochem. 85, 1–26 (2019).
Acknowledgements
X.L. was supported by the National Natural Science Foundation of China (Nos. 42125202 and 41872041). C.T., S.G. and M.M.F. acknowledge funding from the EC Horizon 2020 project European Joint Programme on Radioactive Waste Management (EURAD) under Grant Agreement 847593 (WP FUTURE). Research by C.T. at Lawrence Berkeley National Laboratory (LBNL) was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, through its Geoscience programme at LBNL under Contract DE-AC02-05CH11231. C.T. acknowledges a grant overseen by the French National Research Agency (ANR) as part of the ‘Investissements d’Avenir’ Programme LabEx VOLTAIRE, 10-LABX-0100 at Institut des Sciences de la Terre d’Orléans (ISTO). S.G. acknowledges partial funding by an in-house BRGM grant. A.G.K.acknowledges the financial support of the industrial chair ‘Storage and Disposal of Radioactive Waste’ at the IMT-Atlantique, funded by ANDRA,Orano and EDF.
Author information
Authors and Affiliations
Contributions
X.L. and C.T. were responsible for the design and compilation of the article. All authors contributed to the writing and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Earth & Environment thanks J. Kubicki, B. Sarkar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Clay minerals
-
A class of hydrated phyllosilicates making up the fine-grained fraction of rocks, sediments and soils.
- Metal ions
-
Metal cations (M) in aqueous solution with the chemical formula [M(H2O)n]z+, metal ions include rare earth elements (REEs), actinides, transition metals, and alkaline and alkaline earth metals.
- Earth’s critical zone
-
The thin layer at Earth’s surface and shallow subsurface, spanning from the canopy to the bottom of groundwater, where soil, water, air and the living interact.
- Clay-rich materials
-
Materials containing clay minerals, such as sediments, soils and weathered rocks, and with physical and chemical properties dominated by their clay mineral fraction.
- Complex
-
A compound consisting of a central atom or ion that is bonded to other atoms or ions, which are called ligands.
- Octahedral sheet
-
A 2D sheet formed by octahedral units, each consisting of a metal cation coordinated by six oxygen atoms, linked to six neighbouring octahedra by shared edges.
- Tetrahedral sheets
-
2D sheets formed by tetrahedral units, each consisting of a metal cation coordinated by four oxygen atoms and linked to three neighbouring tetrahedra by shared oxygen.
- 2:1 Layers
-
Structural clay mineral layers made of one octahedral sheet sandwiched by two tetrahedral sheets.
- Epitaxial nucleation
-
The formation of a crystalline nucleus on a substrate, where the new crystalline phase forms with one or more well-defined crystallographic orientations fixed by that of the substrate lattice.
- Neutron diffraction
-
An experimental technique used to probe the crystallographic properties of materials, including the position of hydrogen atoms, notably by taking advantage of the contrasting interactions of neutrons with hydrogen and deuterium.
- Synchrotron X-ray reflectivity
-
An experimental technique used to study the detailed surface properties of solids, based on the analysis of X-rays reflected by a surface.
- X-ray absorption spectroscopy
-
An experimental technique used to study the oxidation state and local environment of an atom in a sample, based on analysis of variations in X-ray absorption over a range of photon energies.
- Smectite
-
A group of 2:1-type clay minerals with expandable interlayer space
- Illite
-
A group of 2:1-type clay minerals with non-expandable interlayer space.
- Inner-sphere complex
-
A complex where the cation is adsorbed on a clay layer with direct chemical contact to the mineral layer surface.
- Outer-sphere complexes
-
Complexes where the cation is adsorbed on a clay layer surface, but is separated by one or more water molecules.
- Ligand exchange
-
A type of reaction in which a ligand of a complex is replaced by a different ligand.
- Dioctahedral
-
A common type of octahedral sheet where most of the metal cations are of +3 valence; two-thirds of the octahedra are occupied whereas the other third is vacant.
Rights and permissions
About this article
Cite this article
Liu, X., Tournassat, C., Grangeon, S. et al. Molecular-level understanding of metal ion retention in clay-rich materials. Nat Rev Earth Environ 3, 461–476 (2022). https://doi.org/10.1038/s43017-022-00301-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43017-022-00301-z
This article is cited by
-
Rare Earths as Emerging Trace Element Contaminants in the Soil
Current Pollution Reports (2024)
-
Biochar-mediated remediation of uranium-contaminated soils: evidence, mechanisms, and perspectives
Biochar (2024)
-
A ferroptosis-targeting ceria anchored halloysite as orally drug delivery system for radiation colitis therapy
Nature Communications (2023)
-
Resolving experimental biases in the interpretation of diffusion experiments with a user-friendly numerical reactive transport approach
Scientific Reports (2023)