Abstract
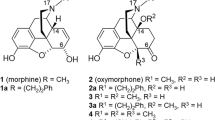
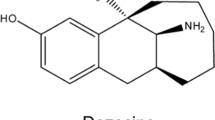
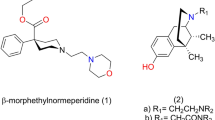
MORPHINE, and its chemical congeners with similar pharmacological activity, tend to fall into a common structural pattern. They are generally tertiary nitrogen bases, −NR3, characterized by the usual sp3 tetrahedral bonding. The actual base function is performed by the unshared pair of electrons on the nitrogen. They have a centrally located, quaternary carbon atom, and an aryl group or groups attached directly to the central carbon atom, and the central carbon is separated from the nitrogen by a two-carbon chain (sp3 bonded).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Eddy, N. B., and Braenden, O. J., Bull. Wld. Hlth. Org., 13, 937 (1955).
Eddy, N. B., Chem. Indust., 1462 (1959).
Harnest, G. H., and Burger, A., J. Amer. Chem. Soc., 65, 370 (1943).
Fellows, E. J., J. Pharmacol. Exp. Therap., 90, 351 (1947).
David, N. A., and Carter, P. C., Proc. West. Pharmacol. Soc., 3, 86 (1960).
Weintraub, L., and Wilson, A., J. Amer. Chem. Soc., 86, 4880 (1964).
Janssen, P. A. J., J. Med. Pharm. Chem., 1, 105 (1959).
Winter, C. A., and Flataker, L., J. Pharmacol. Exp. Therap., 98, 305 (1950).
Eddy, N. B., and Leimbach, D., J. Pharmacol. Exp. Therap., 107, 385 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WILSON, A., PIRCIO, A. Narcotic Analgesics: Possibility of Broadening the Structural Basis of Analgesics. Nature 206, 1151–1152 (1965). https://doi.org/10.1038/2061151a0
Issue Date:
DOI: https://doi.org/10.1038/2061151a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.