Abstract
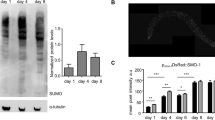
The dauer larva is an alternative larval stage in Caenorhabditis elegans which allows animals to survive through periods of low food availability. Well-fed worms live for about three weeks, but dauer larvae can live for at least two months without affecting post-dauer lifespan1. Mutations in daf-2 and age-1, which produce a dauer constitutive (Daf-C) phenotype, and in clk-1, which are believed to slow metabolism, markedly increase adult lifespan2. Here we show that a ctl-1 mutation reduces adult lifespan in otherwise wild-type animals and eliminates the daf-c and clk-1 -mediated extension of adult lifespan. ctl-1 encodes an unusual cytosolic catalase; a second gene, ctl-2, encodes a peroxisomal catalase. ctl-1 messenger RNA is increased in dauer larvae and adults with the daf-c mutations. We suggest that the ctl-1 catalase is needed during periods of starvation, as in the dauer larva, and that its misexpression in daf-c and clk-1 adults extends lifespan. Cytosolic catalase may have evolved to protect nematodes from oxidative damage produced during prolonged dormancy before reproductive maturity, or it may represent a general mechanism for permitting organisms to cope with the metabolic changes that accompany starvation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Riddle, D. L. & Alberts, P. S. in C. elegans II (eds Riddle, D. L., Blumenthal T., Meyer, B. J. & Priess, J. R.) 739–768 (Cold Spring Harbor Press, New York, (1997).
Kenyon, C. in C. elegans II (eds Riddle, D. L., Blumenthal, T., Meyer, B. J. & Priess, J. R.) 791–814 (Cold Spring Harbor Press, New York, (1997).
Vanfleteren, J. R. Oxidative stress and ageing in Caenorhabditis elegans. Biochem. J. 292, 605–608 (1993).
Waterston, R. et al. Asurvey of expressed genes in Caenorhabditis elegans. Nature Genet. 1, 114–123 (1992).
Gould, S. J., Keller, G.-A., Hosken, N., Wilkinson, J. & Subramani, S. Aconserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 108, 1657–1664 (1989).
Hosokawa, H. et al. Rapid accumulation of fluorescent material with aging in an oxygen-sensitive mutant mev-1 of Caenorhabditis elegans. Mech. Ageing Dev. 74, 161–170 (1994).
Kimura, K. D., Tissenbaum, H. A., Liu, Y. & Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 (1997).
Morris, J. Z., Tissenbaum, H. A. & Ruvkun, G. Aphosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 382, 536–539 (1996).
Ogg, S. et al. The Forkhead transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999 (1997).
Lin, K., Dorman, J. B., Rodan, A. & Kenyon, C. daf-16 : An HNF-3/forkhead family member than can function to double the life-span of Caenorhabditis elegans. Science 278, 1319–1322 (1997).
Larsen, P., Albert, P. S. & Riddle, D. L. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139, 1567–1583 (1995).
Ewbank, J. J. et al. Structural and functional conservation of the Caenorhabditis elegans timing gene clk-1. Science 275, 980–983 (1997).
Beckman, K. B. & Ames, B. N. The free radical theory of aging matures. Physiol. Rev. 78, 547–581 (1998).
Orr, W. C. & Sohal, R. S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263, 1128–1130 (1994).
Amstad, P. et al. The balance between Cu, Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry 30, 9305–9313 (1991).
Parkes, T. L. et al. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nature Genet. 10, 171–175 (1998).
Kirkwood, T. B. L. & Rose, M. R. Evolution of senescence: late survival sacrificed for reproduction. Phil. Trans. R. Soc. Lond. B 332, 15–24 (1991).
Ruis, H. & Koller, F. in Oxidative Stress and the Molecular Biology of Antioxidant Defenses (ed. Scandalios, J. G.) 309–342 (Cold Spring Harbor Press, New York, (1997).
Scandalios, J. G., Guan, L. & Polidoros, A. N. in Oxidative Stress and the Molecular Biology of Antioxidant Defenses (ed. Scandalios, J. G.) 343–406 (Cold Spring Harbor Press, New York, (1997).
Roels, F. Cytochemical demonstration of extraperoxisomal catalase. I. Sheep liver. J.. Histochem. Cytochem. 24, 713–724 (1976).
Roels, F., de Coster, W. & Goldfischer, S. Cytochemical demonstration of extraperoxisomal catalase. II. Liver of rhesus monkey and guinea pig. J. Histochem. Cytochem. 25, 157–160 (1977).
Yamamoto, K., Volkl, A., Hashimoto, T. & Fahimi, H. D. Catalase in guinea pig hepatocytes is localized in cytoplasm, nuclear matrix and peroxisomes. Eur. J. Cell Biol. 46, 129–135 (1988).
Bulitta, C., Ganea, C., Fahimi, H. D. & Volkl, A. Cytoplasmic and peroxisomal catalases of the guinea pig liver: evidence for two distinct proteins. Biochim. Biophys. Acta 1293, 55–62 (1996).
Bissinger, P. H., Wieser, R., Hamilton, B. & Ruis, H. Control of Saccharomyces cerevisiae catalase T gene (CTT1) expression by nutrient supply via the RAS-cyclic AMP pathway. Mol. Cell Biol. 9, 1309–1315 (1989).
Loewen, P. in Oxidative Stress and the Molecular Biology of Antioxidant Defenses (ed. Scandalios, J. G.) 273–308 (Cold Spring Harbor Press, New York, (1997).
Masoro, E. J., Shimokawa, I. & Yu, B. P. Retardation of the aging processes in rats by food restriction. Ann. N^Y Acad. Sci. 621, 337–352 (1991).
Sulston, J. & Hodgkin, J. in The Nematode Caenorhabditis elegans (ed. Wood, W. B.) 587–606 (Cold Spring Harbor Press, New York, (1988).
Peters, T. J., Muller, M. & DeDuve, C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J. Exp. Med. 136, 1117–1139 (1972).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: A Laboratory Manual 2nd edn (Cold Spring Harbor Laboratory Press, New York, (1989).
Mello, C. & Fire, A. Methods Cell Biol. 48, 451–482 (1995).
Acknowledgements
This work was supported by the NIH (M.C. and J.R.) and by the American Cancer Society (J.R.). Some strains were received from the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taub, J., Lau, J., Ma, C. et al. A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-C and clk-1 mutants. Nature 399, 162–166 (1999). https://doi.org/10.1038/20208
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/20208
This article is cited by
-
Single swim sessions in C. elegans induce key features of mammalian exercise
BMC Biology (2017)
-
Epigenetic Silencing of the Key Antioxidant Enzyme Catalase in Karyotypically Abnormal Human Pluripotent Stem Cells
Scientific Reports (2016)
-
p66Shc as a switch in bringing about contrasting responses in cell growth: implications on cell proliferation and apoptosis
Molecular Cancer (2015)
-
MnSOD activity regulates hydroxytyrosol-induced extension of chronological lifespan
AGE (2012)
-
FoxO3a Changes in Pyramidal Neurons and Expresses in Non-Pyramidal Neurons and Astrocytes in the Gerbil Hippocampal CA1 Region After Transient Cerebral Ischemia
Neurochemical Research (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.