Abstract
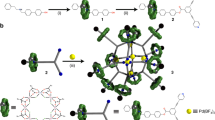
Molecular capsules consist of closed, hollow frameworks within which encapsulated molecules are isolated from interaction with external molecules1. In this environment, otherwise reactive molecules can be stabilized2,3,4,5. Although some molecular capsules have been prepared by conventional synthetic chemistry1, recent progress in non-covalent synthesis has allowed the creation of capsules held together by hydrogen bonds6,7,8,9. Here we report the use of transition-metal-based coordination chemistry10,11,12,13,14,15,16,17,18,19 to assemble a stable, nanometre-scale capsule from 24 small components: 18 metal ions and six triangular ligands. The capsule is roughly hexahedral and comprises six edge-sharing triangles with two metal ions on each edge. The internal space has a volume of 900 Å3 and is fully closed to all but very small molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cram, D. J. & Cram, JM. Container Molecules and their Guests (Royal Society of Chemistry, Cambridge, UK, (1994).
Cram, D. J., Tanner, M. E. & Thomas, R. The taming of cyclobutadiene. Angew. Chem. Int. Edn Engl. 30, 1024–1027 (1991).
Warmuth, R. o -Benzyne: strained alkyne or cumulene?—NMR characterization in a molecular container. Angew. Chem. Int. Edn Engl. 36, 1347–1350 (1997).
Kang, J. & Rebek, J. J Acceleration of a Diels-Alder reaction by a self-assembled molecular capsule. Nature 385, 50–52 (1997).
Kang, J., Santamaría, J., Hilmersson, G. & Rebek, J. J Self-assembled molecular capsule catalyzes a Diels-Alder reaction. J. Am. Chem. Soc. 120, 7389–7390 (1998).
Wyler, R., de Mendoza, J. & Rebek, J. J Asynthetic cavity assembles through self-complementary hydrogen bonds. Angew. Chem. Int. Edn Engl. 32, 1699–1701 (1993).
Rebek, J. J Assembly and encapsulation with self-complementary molecules. Chem. Soc. Rev. 25, 255–264 (1996).
Heinz, T., Rudlkevich, D. M. & Rebek, J. J Pairwise selection of guests in a cylindrical molecular capsule of nanometre dimensions. Nature 394, 764–766 (1998).
MacGillivray, L. R. & Atwood, J. L. Achiral spherical molecular assembly held together by 60 hydrogen bonds. Nature 389, 469–472 (1997).
Saalfrank, R. W., Stark, A., Peters, K. & von Schnering, H. G. The first “adamantoid” alkaline earth metal chelate complex: synthesis, structure, and reactivity. Angew. Chem. Int. Edn Engl. 27, 851–853 (1988).
Boxter, P., Lehn, J.-M., DeCian, A. & Fischer, J. Multicomponent self-assembly: spontaneous formation of a cylindrical complex from five ligands and six metal ions. Angew. Chem. Int. Edn Engl. 32, 69–72 (1993).
Fujita, M.et al. Self-assembly of ten molecules into nanometre-sized organic host frameworks. Nature 378, 469– 471 (1995).
Beissel, T., Power, R. E. & Raymond, K. N. Symmetry-based metal complex cluster formation. Angew. Chem. Int. Edn Engl. 35, 1084– 1086 (1996).
Hartshorn, C. M. & Steel, P. J. Self-assembly and X-ray structure of a ten-component, three-dimensional metallosupramolecular cage. Chem. Commun. 541–542 (1997).
Ibukuro, F., Kusukawa, T. & Fujita, M. Athermally switchable molecular lock. Guest-templated synthesis of a kinetically stable nanosized cage. J. Am. Chem. Soc. 120, 8561–8562 ( 1998).
Kusukawa, T. & Fujita, M. Encapsulation of large, neutral molecules in a self-assembled nanocage incorporating six palladium(II) ions. Angew. Chem. Int. Edn Engl. 37, 3142– 3144 (1998).
Fujita, M. in Comprehensive Supramolecular Chemistry Vol. 9(eds Sauvage, J.-P. & Hosseini, M. W.) 253–282 (Pergamon, Oxford, (1996).
Stang, P. J. & Olenyuk, B. Self-assembly, symmetry, and molecular architecture: coordination as the motif in the rational design of supramolecular metallacyclic polygons and polyhedra. Acc. Chem. Res. 30, 502–518 (1997).
Fujita, M. Metal-directed self-assembly of two- and three-dimensional synthetic receptors. Chem. Soc. Rev. 27, 417– 425 (1998).
Fujita, M., Yazaki, J. & Ogura, K. Preparation of a macrocyclic polynuclear complex, [(en)Pd(4,4′-bpy)] 4(NO3)8, which recognizes an organic molecule in aqueous media. J. Am. Chem. Soc. 112, 5645–5647 (1990).
Fujita, M. & Ogura, K. Supramolecular self-assembly of macrocycles, catenanes, and cages through coordination of pyridine-based ligands to transition metals. Bull. Chem. Soc. Jpn 69, 1471– 1482 (1996).
Acknowledgements
We thank K. Biradha for part of the crystallographic studies.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Takeda, N., Umemoto, K., Yamaguchi, K. et al. A nanometre-sized hexahedral coordination capsule assembled from 24 components . Nature 398, 794–796 (1999). https://doi.org/10.1038/19734
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/19734
This article is cited by
-
The sharp structural switch of covalent cages mediated by subtle variation of directing groups
Nature Communications (2023)
-
Post-synthetic modifications of metal–organic cages
Nature Reviews Chemistry (2022)
-
A metal–peptide capsule by multiple ring threading
Nature Communications (2019)
-
The applications of small-angle X-ray scattering in studying nano-scaled polyoxometalate clusters in solutions
Journal of Nanoparticle Research (2018)
-
A Cobalt Supramolecular Triple-Stranded Helicate-based Discrete Molecular Cage
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.