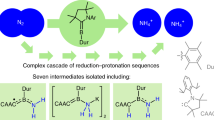
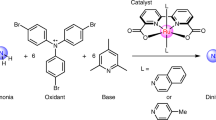
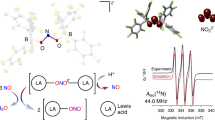
Abstract
REACTION of trivalent nitrogen with the azide ion, usually called the ‘nitrite-azide reaction’, is generally known and has been a subject of recent detailed investigations1–4. This azide reaction has been considered to be specific for trivalent nitrogen even in strongly acidic media, and the fact that certain reactions of pentavalent nitrogen (for example, oxidation processes of nitric acid, lead chamber process) are stopped by the addition of azide ions has been accepted as a proof that they proceed through the trivalent nitrogen5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Seel, F., and Schwaebel, D., Z. anorg. allg. Chem., 274, 169 (1953).
Seel, F., Wölfle, R., and Zwarg, G., Z. Naturforsch., 13, b, 136 (1958).
Stedman, G., J. Chem. Soc., 2943, 2949 (1959); 1702 (1960).
Bunton, C. A., and Stedman, G., J. Chem. Soc., 3466 (1959).
Seel, F., Angew. Chem., 68, 272 (1956).
Mašek, J., Advances in Polarography, 1, 340 (Pergamon Press, London, 1960).
Mašek, J., Coll. Czechaslov. Chem. Comm., 25, 3137 (1960).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MAŠEK, J. A New Inorganic Reaction: Nitric Acid–Hydrazoic Acid. Nature 191, 166–167 (1961). https://doi.org/10.1038/191166a0
Issue Date:
DOI: https://doi.org/10.1038/191166a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.