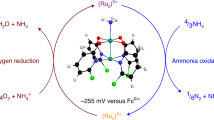
Abstract
Conversion of ammonia into dinitrogen has attracted broad scientific interest in relation to molecular models of the heterogeneous nitrogen fixation process, environmental treatment for denitrification and utilization of ammonia as an energy carrier. Here we show that some ruthenium complexes bearing 2,2′-bipyridyl-6,6′-dicarboxylate ligands work as catalysts for the ammonia oxidation reaction. Production of dinitrogen is observed when ammonium salts are treated with a triarylaminium radical as an oxidant and 2,4,6-collidine as a base in the presence of the ruthenium catalysts. Based on the characterization of some intermediates, we propose a reaction pathway via a bimetallic nitride–nitride coupling process. The proposed reaction pathway is supported by density functional theory calculations. Further investigation of the ammonia oxidation reaction under the electrochemical conditions revealed that the ruthenium complex works as a new anode catalyst for ammonia oxidation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for the structure reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition nos. CCDC 1860066 (6a), 1860067 (6b), 1860068 (7), 1860069 (8) and 1860070 (9) (Supplementary Table 5). All other data supporting the findings of this study are available within the Article and its Supplementary Information, or from the corresponding author upon reasonable request.
Change history
26 February 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Guo, J. & Chen, P. Catalyst: NH3 as an energy carrier. Chem. 3, 709–712 (2017).
Giddy, S., Badwal, S. P. S., Munnings, C. & Dolan, M. Ammonia as a renewable energy transportation media. ACS Sustain. Chem. Eng. 5, 10231–10239 (2017).
Lan, R., Irvine, J. T. S. & Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 37, 1482–1494 (2012).
Adli, N. M., Zhang, H., Mukherjee, S. & Wu, G. Review—ammonia oxidation electrocatalysis for hydrogen generation and fuel cells. J. Electrochem. Soc. 165, J3130–J3147 (2018).
Mukherjee, S. et al. Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl. Catal. B Environ. 226, 162–181 (2018).
Zhong, C., Hu, W. B. & Cheng, Y. F. Recent advances in electrocatalysts for electro-oxidation of ammonia. J. Mater. Chem. A 1, 3216–3238 (2013).
Habibzadeh, F., Miller, S. L., Hamann, T. W. & Smith, M. R. III Homogeneous electrocatalytic oxidation of ammonia to N2 under mild conditions. Proc. Natl Acad. Sci. USA 116, 2849–2853 (2019).
Pipes, D. W. et al. Reversible interconversion between a nitrido complex of Os(vi) and an ammino complex of osmium(ii). J. Am. Chem. Soc. 112, 5507–5514 (1990).
Ishitani, O., White, P. S. & Meyer, T. J. Formation of dinitrogen by oxidation of [(bpy)2(NH3)RuORu(NH3)(bpy)2]4+. Inorg. Chem. 35, 2167–2168 (1996).
Ishitani, O., Ando, E. & Meyer, T. J. Dinitrogen formation by oxidative intramolecular N–N coupling in cis,cis-[(bpy)2(NH3)RuORu(NH3)(bpy)2]4+. Inorg. Chem. 42, 1707–1710 (2003).
Bezdek, M. J., Guo, S. & Chirik, P. J. Coordination-induced weakening of ammonia, water, and hydrazine X–H bonds in a molybdenum complex. Science 354, 730–733 (2016).
Margulieux, G. W., Bezdek, M. J., Turner, Z. R. & Chirik, P. J. Ammonia activation, H2 evolution and nitride formation from a molybdenum complex with a chemically and redox noninnocent ligand. J. Am. Chem. Soc. 139, 6110–6113 (2017).
Bhattacharya, P. et al. Ammonia oxidation by abstraction of three hydrogen atoms from a Mo–NH3 complex. J. Am. Chem. Soc. 139, 2916–2919 (2017).
Man, W.-L., Lam, W. W. Y. & Lau, T.-C. Reactivity of nitrido complexes of ruthenium(vi), osmium(vi) and manganese(v) bearing Schiff base and simple anionic ligands. Acc. Chem. Res. 47, 427–439 (2014).
Berry, J. F. Terminal nitrido and imido complexes of the late transition metals. Comments Inorg. Chem. 30, 28–66 (2009).
Man, W.-L. et al. Highly electrophilic (salen)ruthenium(vi) nitrido complexes. J. Am. Chem. Soc. 126, 478–479 (2004).
Betley, T. A. & Peters, J. C. A tetrahedrally coordinated L3Fe-Nx platform that accommodates terminal nitride (FeIV≡N) and dinitrogen (FeI–N2–FeI) ligands. J. Am. Chem. Soc. 126, 6252–6254 (2004).
Scheibel, M. G. et al. Closed-shell and open-shell square-planar iridium nitrido complexes. Nat. Chem. 4, 552–558 (2012).
Miyazaki, T. et al. Cleavage and formation of molecular dinitrogen in a single system assisted by molybdenum complexes bearing ferrocenyldiphosphine. Angew. Chem. Int. Ed. 53, 11488–11492 (2014).
Clarke, R. M. & Storr, T. Tuning electronic structure to control manganese nitride activation. J. Am. Chem. Soc. 138, 15299–15302 (2016).
Keener, M. et al. Towards catalytic ammonia oxidation to dinitrogen: a synthetic cycle by using a simple manganese complex. Chem. Eur. J. 23, 11479–11484 (2017).
Duan, L. et al. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 4, 418–423 (2012).
Gersten, S. W., Samuels, G. J. & Meyer, T. J. Catalytic oxidation of water by an oxo-bridged ruthenium dimer. J. Am. Chem. Soc. 104, 4029–4030 (1982).
Tseng, H.-W., Zong, R., Muckerman, J. T. & Thummel, R. Mononuclear ruthenium(ii) complexes that catalyze water oxidation. Inorg. Chem. 47, 11763–11773 (2008).
Masaoka, S. & Sakai, K. Clear evidence showing the robustness of a highly active oxygen-evolving mononuclear ruthenium complex with an aqua ligand. Chem. Lett. 38, 182–183 (2009).
Zhang, B. et al. Characterization of a trinuclear ruthenium species in catalytic water oxidation by Ru(bda)(pic)2 in neutral media. Chem. Commun. 52, 8619–8622 (2016).
Duan, L., Fischer, A., Xu, Y. & Sun, L. Isolated seven-coordinate Ru(iv) dimer complex with [HOHOH]– bridging ligand as an intermediate for catalytic water oxidation. J. Am. Chem. Soc. 131, 10397–10399 (2009).
MacKay, B. A. & Fryzuk, M. D. Dinitrogen coordination chemistry: on the biomimetic borderlands. Chem. Rev. 104, 385–402 (2004).
Huynh, M. H. V. & Meyer, T. J. Proton-coupled electron transfer. Chem. Rev. 107, 5004–5064 (2007).
Waidmann, C. R. et al. Using combinations of oxidants and bases as PCET reactants: thermochemical and practical considerations. Energy Environ. Sci. 5, 7771–7780 (2012).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. & Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Coia, G. M. et al. Osmium hydrazido and dinitrogen complexes. Inorg. Chem. 36, 2341–2351 (1997).
Lindley, B. M. et al. Evaluating the thermodynamics of electrocatalytic N2 reduction in acetonitrile. ACS Energy Lett. 1, 698–704 (2016).
Pavlishchuk, V. V. & Addison, A. W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorg. Chim. Acta 298, 97–102 (2000).
Nicholson, R. S. & Shain, I. Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal. Chem. 36, 706–723 (1964).
Rees, N. V. & Compton, R. G. Carbon-free energy: a review of ammonia- and hydrazine-based electrochemical fuel cells. Energy Environ. Sci. 4, 1255–1260 (2011).
Ertl, G. Reactions at surfaces: from atoms to complexity (Nobel lecture). Angew. Chem. Int. Ed. 47, 3524–3535 (2008).
Du, R. et al. Advanced nitrogen removal from wastewater by combining anammox with partial denitrification. Bioresour. Technol. 179, 497–504 (2015).
Acknowledgements
This work was supported by CREST, JST (JPMJCR1541). The authors acknowledge support from JSPS KAKENHI grants nos. JP15H05798, JP17H01201, JP18K19093 and JP19K15556 from the Japan Society for the Promotion of Science (JSPS) and the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT). We also thank J. C. Peters and S. Schneider for helpful discussions.
Author information
Authors and Affiliations
Contributions
K.S. and Y.N. directed and conceived this project. K.N. and H.T. conducted the experimental work. K.S. conducted the computational work. All authors discussed the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
K.N., H.T. and Y.N. have filed a patent based on the work described here (Japanese patent application no. 2018-036966).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures; products characterization; X-ray crystallographic analysis; kinetic, electrochemical and DFT studies.
6a-CH4O.cif
Crystallographic Information File for compounds 6a, CCDC 1860066
6b-2CH4O.cif
Crystallographic Information File for compounds 6b, CCDC 1860067
7-H2O.cif
Crystallographic Information File for compounds 7, CCDC 1860068
8.cif
Crystallographic Information File for compounds 8, CCDC 1860069
9.cif
Crystallographic Information File for compounds 9, CCDC 1860070
Rights and permissions
About this article
Cite this article
Nakajima, K., Toda, H., Sakata, K. et al. Ruthenium-catalysed oxidative conversion of ammonia into dinitrogen. Nat. Chem. 11, 702–709 (2019). https://doi.org/10.1038/s41557-019-0293-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0293-y
This article is cited by
-
Direct synthesis of hydrazine by efficient electrochemical ruthenium-catalysed ammonia oxidation
Nature Catalysis (2023)
-
Regulation of the electrocatalytic nitrogen cycle based on sequential proton–electron transfer
Nature Catalysis (2022)
-
Computational Investigations of the Reactivity of Metalloporphyrins for Ammonia Oxidation
Topics in Catalysis (2022)
-
The Role of Counterions in Intermolecular Radical Coupling of Ru-bda Catalysts
Topics in Catalysis (2022)
-
Spontaneous N2 formation by a diruthenium complex enables electrocatalytic and aerobic oxidation of ammonia
Nature Chemistry (2021)