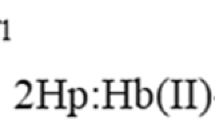Abstract
IF, in a hæmoprotein, the hæm iron atom is held by bonds to the protein on both sides of the porphyrin ring, then the most likely mechanism for complex formation with the ligands CO, NO, F−, CN−, N3 −, etc., entails breaking the weaker bond while leaving the stronger bond intact. The proton affinity of the group thus liberated would be an important factor in determining the pH variation of the equilibrium constants for complex formation. The limited extent to which such a mechanism can account for hæm-linked ionizations in hæmoglobin reactions, its direct application to cytochrome c reactions, and the way it can account for some of the differences found in peroxidase and catalase reactions have been discussed recently1,2. The question naturally arises whether any other functional groups in amino-acid side-chains can co-ordinate with the iron beside nitrogenous base groups, which in cytochrome c give rise to the characteristic hæmochromogen and parahæmatin spectra that set it apart from the other ferro- and ferri-hæmoproteins. Internal complex formation is a possibility in the ferric oxidation state via—COOH, alcoholic —OH and —SH groups in the side-chains, since complexes are known to be formed between ferrihæmoglobin and formic acid, acetic acid, propionic acid, ethanol, hydrogen sulphide and ethyl mercaptan3–6. Furthermore, the following preliminary report on the reaction of ferrimyoglobin with phenols shows that the phenolic —OH group can be added to this list.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
George, P., and Lyster, R. L. J., in “Conference on Hemoglobin, May 2–3” (Washington, National Academy of Sciences—National Research Council, 1957) (NAS–NRC Publication 557) (in the press).
George, P., and Lyster, R. L. J., Fed. Proc., 16, 1051 (1957).
Scheler, W., Salewski, A., and Jung, F., Biochem. Z., 326, 288 (1955).
Coryell, C. D., and Stitt, F., J. Amer. Chem. Soc., 62, 2942 (1940).
Keilin, D., Proc. Roy. Soc., B, 113, 393 (1933).
Coryell, C. D., and Heussenstamm, P., see Coryell, C. D., in “Chemical Specificity in Biological Interactions”, pp. 108 and 113 (Academic Press, Inc., New York, 1954).
Theorell, H., Ark. Kemi Min. Geol., 16A, No. 3 (1942).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GEORGE, P., LYSTER, R. & BEETLESTONE, J. Reaction of Ferrimyoglobin with Phenols. Nature 181, 1534–1535 (1958). https://doi.org/10.1038/1811534a0
Issue Date:
DOI: https://doi.org/10.1038/1811534a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.